Textbook Question
Write a balanced chemical equation for the formation of a polymer via a condensation reaction from the monomers succinic acid 1HOOCCH2CH2COOH2 and ethylenediamine 1H2NCH2CH2NH22.

 Verified step by step guidance
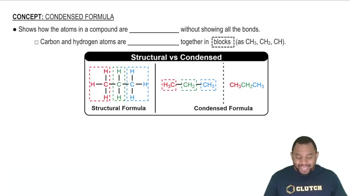
Verified step by step guidance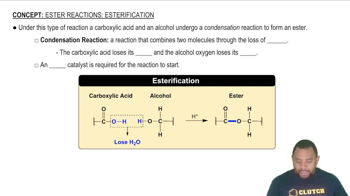

Write a balanced chemical equation for the formation of a polymer via a condensation reaction from the monomers succinic acid 1HOOCCH2CH2COOH2 and ethylenediamine 1H2NCH2CH2NH22.
Write the chemical equation that represents the formation of (b) polyacrylonitrile from acrylonitrile (polyacrylonitrile is used in home furnishings, craft yarns, clothing, and many other items).