Textbook Question
For each of the following pairs of semiconductors, which one will have the larger band gap: (a) CdS or CdTe? (b) GaN or InP? (c) GaAs or InAs?

 Verified step by step guidance
Verified step by step guidance

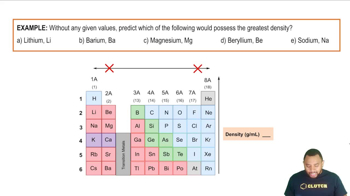
For each of the following pairs of semiconductors, which one will have the larger band gap: (a) CdS or CdTe? (b) GaN or InP? (c) GaAs or InAs?
If you want to dope GaAs to make an n-type semiconductor with an element to replace Ga, which element(s) would you pick?
Cadmium telluride is an important material for solar cells. (b) What wavelength of light would a photon of this energy correspond to?
Cadmium telluride is an important material for solar cells. (d) With respect to silicon, does CdTe absorb a larger or smaller portion of the solar spectrum?