Repeat Exercise 12.51 for a linear chain of eight lithium atoms. (f) How does the HOMO–LUMO energy gap for this case compare to that of the four-atom case?
Ch.12 - Solids and Modern Materials

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 12, Problem 55
Arrange the following metals in increasing order of expected melting points: Mo, Zr, Y, Nb. Explain this trend in melting points.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of melting points in metals. Melting points are influenced by the strength of metallic bonds, which depend on factors such as atomic size, electron configuration, and the number of valence electrons.
Step 2: Consider the position of each metal in the periodic table. Molybdenum (Mo), Zirconium (Zr), Yttrium (Y), and Niobium (Nb) are all transition metals, and their properties can be compared based on their group and period.
Step 3: Analyze the electron configuration of each metal. Transition metals have partially filled d-orbitals, and the number of electrons in these orbitals can affect the strength of metallic bonding.
Step 4: Compare the atomic sizes of the metals. Generally, smaller atoms can pack more closely together, leading to stronger metallic bonds and higher melting points.
Step 5: Arrange the metals based on the analysis of their atomic size, electron configuration, and position in the periodic table to determine the trend in melting points.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Melting Point Trends in Metals
The melting point of metals is influenced by their atomic structure and bonding. Generally, metals with stronger metallic bonds, which arise from a higher number of delocalized electrons, exhibit higher melting points. Transition metals, like those in the question, often have varying melting points due to differences in their electron configurations and crystal lattice structures.
Recommended video:
Guided course
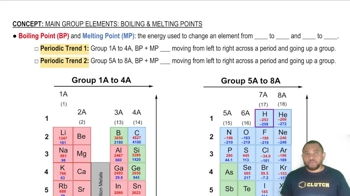
Boiling Point and Melting Point
Transition Metals
Transition metals are elements found in the d-block of the periodic table, characterized by their ability to form variable oxidation states and complex ions. They typically have high melting points due to the presence of d-electrons that contribute to metallic bonding. The specific arrangement of these d-electrons can significantly affect the melting point of each metal.
Recommended video:
Guided course
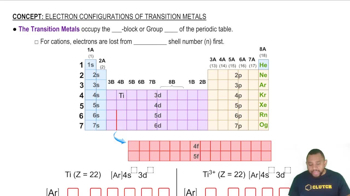
Transition Metals
Periodic Trends
Periodic trends refer to the predictable patterns observed in the properties of elements as you move across or down the periodic table. For melting points, as you move from left to right across a period, melting points generally increase due to stronger bonding. However, this trend can vary among groups, especially in transition metals, where factors like atomic size and electron configuration play critical roles.
Recommended video:
Guided course
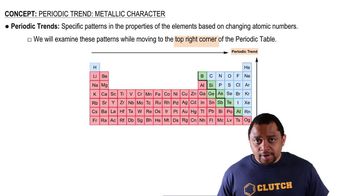
Periodic Trends
Related Practice
Textbook Question
Textbook Question
Which would you expect to be the more ductile element, (a) Ag or Mo? (b) Zn or Si? In each case explain your reasoning.
Textbook Question
Which of the following statements does not follow from the fact that the alkali metals have relatively weak metal–metal bonding? (a) The alkali metals are less dense than other metals. (b) The alkali metals are soft enough to be cut with a knife. (c) The alkali metals are more reactive than other metals. (d) The alkali metals have higher melting points than other metals. (e) The alkali metals have low ionization energies.
Textbook Question
For each of the following groups, which metal would you expect to have the highest melting point: (b) rubidium, molybdenum, or indium?
4
views
Textbook Question
Tausonite, a mineral composed of Sr, O, and Ti, has the cubic unit cell shown in the drawing. (a) What is the empirical formula of this mineral?
Textbook Question
The unit cell of a compound containing Co and O has a unitcell shown below. The Co atoms are on the corners, and the Oatoms are completely within the unit cell. What is the empiricalformula of this compound? What is the oxidation state ofthe metal?
