Name the phase transition in each of the following situations and indicate whether it is exothermic or endothermic: (c) Rubbing alcohol in an open container slowly disappears.

Ethyl chloride (C2H5Cl) boils at 12 °C. When liquid C2H5Cl under pressure is sprayed on a room-temperature (25 °C) surface in air, the surface is cooled considerably. (a) What does this observation tell us about the specific heat of C2H5Cl(g) as compared with that of C2H5Cl(l)?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
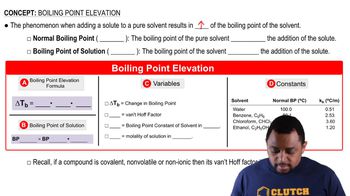
Phase Change and Boiling Point
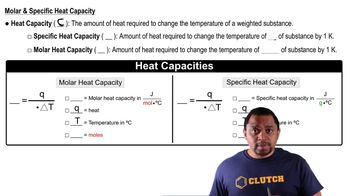
Specific Heat Capacity
Latent Heat of Vaporization

Name the phase transition in each of the following situations and indicate whether it is exothermic or endothermic: (d) Molten lava from a volcano turns into solid rock.
Ethyl chloride (C2H5Cl) boils at 12 °C. When liquid C2H5Cl under pressure is sprayed on a room-temperature (25 °C) surface in air, the surface is cooled considerably. (b) Assume that the heat lost by the surface is gained by ethyl chloride. What enthalpies must you consider if you were to calculate the final temperature of the surface?
For many years drinking water has been cooled in hot climates by evaporating it from the surfaces of canvas bags or porous clay pots. How many grams of water can be cooled from 35 to 20 °C by the evaporation of 60 g of water? (The heat of vaporization of water in this temperature range is 2.4 kJ/g. The specific heat of water is 4.18 J/g-K).
