At room temperature, Si is a solid, CCl4 is a liquid, and Ar is a gas. List these substances in order of (a) increasing intermolecular energy of attraction
Ch.11 - Liquids and Intermolecular Forces

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 11, Problem 13
At standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively. (a) Given the different molecular weights, dipole moments, and molecular shapes, why are their molar volumes nearly the same?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Begin by understanding the concept of molar volume. Molar volume is the volume occupied by one mole of a substance at a given temperature and pressure. For gases at standard temperature and pressure (STP), the molar volume is approximately 22.4 L/mol.
Step 2: Consider the ideal gas law, which states that PV = nRT. At STP, the pressure (P) is 1 atm, the temperature (T) is 273.15 K, and the gas constant (R) is 0.0821 L·atm/mol·K. This equation implies that the molar volume of an ideal gas is determined by these conditions, not by the properties of the gas itself.
Step 3: Recognize that Cl2 and NH3 are both gases and, under STP conditions, they behave similarly to ideal gases. Despite differences in molecular weight, dipole moments, and molecular shapes, their molar volumes are close to the ideal gas molar volume due to the dominance of the ideal gas behavior at STP.
Step 4: Analyze the molecular characteristics: Cl2 is a nonpolar diatomic molecule with a linear shape, while NH3 is a polar molecule with a trigonal pyramidal shape. These differences affect intermolecular forces but have minimal impact on molar volume at STP.
Step 5: Conclude that the nearly identical molar volumes of Cl2 and NH3 at STP are primarily due to the conditions of STP, where gases exhibit ideal behavior, rather than their molecular properties. The ideal gas law provides a good approximation for molar volume under these conditions.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Volume
Molar volume is the volume occupied by one mole of a substance at a given temperature and pressure, typically measured in liters per mole. At standard temperature and pressure (STP), the molar volume of an ideal gas is approximately 22.4 L. However, real gases can deviate from this ideal behavior due to intermolecular forces and molecular size, which can affect their actual molar volumes.
Recommended video:
Guided course
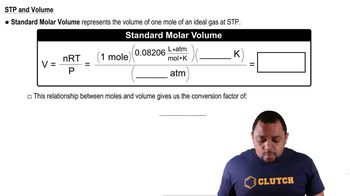
Standard Molar Volume
Intermolecular Forces
Intermolecular forces are the forces of attraction or repulsion between molecules, which can significantly influence the physical properties of substances, including their molar volumes. For gases like Cl2 and NH3, the presence of dipole moments and hydrogen bonding in NH3 leads to stronger intermolecular interactions compared to the nonpolar Cl2. However, at STP, these forces may not drastically alter the molar volumes of the gases, resulting in their similar values.
Recommended video:
Guided course

Intermolecular vs Intramolecular Forces
Molecular Weight and Shape
Molecular weight and shape play crucial roles in determining the behavior of gases. While Cl2 has a higher molecular weight than NH3, the shape and size of the molecules also affect how they occupy space. The linear shape of Cl2 allows for efficient packing, while the trigonal pyramidal shape of NH3, despite its lower molecular weight, can lead to similar molar volumes due to the balance of molecular interactions and the available space in the gas phase.
Recommended video:
Guided course

Molecular Shapes and VSEPR
Related Practice
Textbook Question
3
views
Textbook Question
At standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively (b) On cooling to 160 K, both substances form crystalline solids. Do you expect the molar volumes to decrease or increase on cooling the gases to 160 K?
Textbook Question
At standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively. (c) The densities of crystalline Cl2 and NH3 at 160 K are 2.02 and 0.84 g/cm3, respectively. Calculate their molar volumes.
Textbook Question
At standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively (d) Are the molar volumes in the solid state as similar as they are in the gaseous state? Explain.
1
views
