The phase diagram for neon is
Use the phase diagram to answer the following questions. (a) What is the approximate value of the normal melting point?

 Verified step by step guidance
Verified step by step guidance


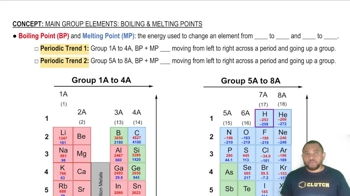
The phase diagram for neon is
Use the phase diagram to answer the following questions. (a) What is the approximate value of the normal melting point?
Use the phase diagram of neon to answer the following questions. (a) What is the approximate value of the normal boiling point?
Use the phase diagram of neon to answer the following questions. (b) What can you say about the strength of the intermolecular forces in neon and argon based on the critical points of Ne and Ar (see Table 11.5.)?
Indicate whether each statement is true or false: (a) The liquid crystal state is another phase of matter, just like solid, liquid, and gas. (b) Liquid crystalline molecules are generally spherical in shape. (d) Molecules that exhibit a liquid crystalline phase show weaker-than-expected intermolecular forces. (e) Molecules containing only carbon and hydrogen are likely to form liquid crystalline phases. (f) Molecules can exhibit more than one liquid crystalline phase.