A particular liquid crystalline substance has the phase diagram shown in the figure. By analogy with the phase diagram for a nonliquid crystalline substance, identify the phase present in each area.

Acetone [(CH3)2CO] is widely used as an industrial solvent. (d) 1-Propanol (CH3CH2CH2OH) has a molecular weight that is very similar to that of acetone, yet acetone boils at 56.5 °C and 1-propanol boils at 97.2 °C. Explain the difference.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Intermolecular Forces
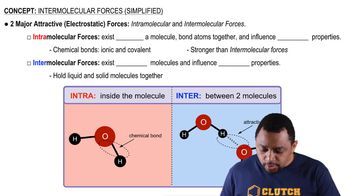
Molecular Structure and Polarity
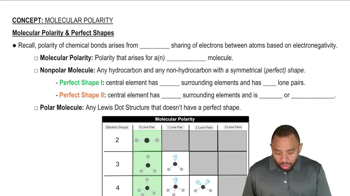
Boiling Point and Energy Requirements
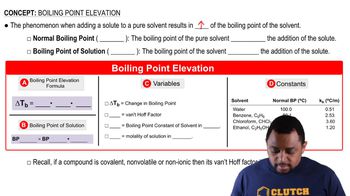
In Table 11.3, we saw that the viscosity of a series of hydrocarbons increased with molecular weight, doubling from the six-carbon molecule to the ten-carbon molecule.
(a) The eight-carbon hydrocarbon, octane, has an isomer, isooctane. Would you predict that isooctane would have a larger or smaller viscosity than octane? Why?
The vapor pressure of ethanol (C2H5OH) at 19 °C is 40.0 torr. A 1.00-g sample of ethanol is placed in a 2.00 L container at 19 °C. If the container is closed and the ethanol is allowed to reach equilibrium with its vapor, how many grams of liquid ethanol remain?
Using information in Appendices B and C, calculate the minimum grams of propane, C3H8(g), that must be combusted to provide the energy necessary to convert 5.50 kg of ice at -20 °C to liquid water at 75 °C
