
A particular liquid crystalline substance has the phase diagram shown in the figure. By analogy with the phase diagram for a nonliquid crystalline substance, identify the phase present in each area.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Phase Diagrams

Liquid Crystals
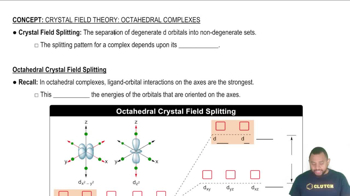
Phase Transitions

Naphthalene (C10H8) is the main ingredient in traditional mothballs. Its normal melting point is 81 °C, its normal boiling point is 218 °C, and its triple point is 80 °C at 1000 Pa. Using the data, construct a phase diagram for naphthalene, labeling all the regions of your diagram.
In Table 11.3, we saw that the viscosity of a series of hydrocarbons increased with molecular weight, doubling from the six-carbon molecule to the ten-carbon molecule.
(a) The eight-carbon hydrocarbon, octane, has an isomer, isooctane. Would you predict that isooctane would have a larger or smaller viscosity than octane? Why?
The vapor pressure of ethanol (C2H5OH) at 19 °C is 40.0 torr. A 1.00-g sample of ethanol is placed in a 2.00 L container at 19 °C. If the container is closed and the ethanol is allowed to reach equilibrium with its vapor, how many grams of liquid ethanol remain?
