Which one or more of the following statements are true? (a) O2 will effuse faster than Cl2. (b) Effusion and diffusion are different names for the same process. (c) Perfume molecules travel to your nose by the process of effusion. (d) The higher the density of a gas, the shorter the mean free path.

As discussed in the “Chemistry Put to Work” box in Section 10.8, enriched uranium can be produced by effusion of gaseous UF6 across a porous membrane. Suppose a process were developed to allow effusion of gaseous uranium atoms, U(g). Calculate the ratio of effusion rates for 235U and 238U, and compare it to the ratio for UF6 given in the essay.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
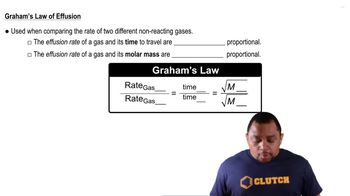
Graham's Law of Effusion
Isotopes and Molar Mass

Porous Membrane and Effusion
At constant pressure, the mean free path 1l2 of a gas molecule is directly proportional to temperature. At constant temperature, l is inversely proportional to pressure. If you compare two different gas molecules at the same temperature and pressure, l is inversely proportional to the square of the diameter of the gas molecules. Put these facts together to create a formula for the mean free path of a gas molecule with a proportionality constant (call it Rmfp, like the ideal-gas constant) and define units for Rmfp.
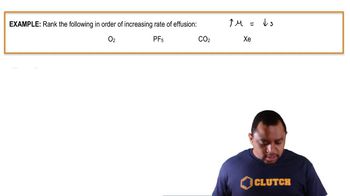
Hydrogen has two naturally occurring isotopes, 1H and 2H. Chlorine also has two naturally occurring isotopes, 35Cl and 37Cl. Thus, hydrogen chloride gas consists of four distinct types of molecules: 1H35Cl, 1H37Cl, 2H35Cl, and 2H37Cl. Place these four molecules in order of increasing rate of effusion.
Arsenic(III) sulfide sublimes readily, even below its melting point of 320 °C. The molecules of the vapor phase are found to effuse through a tiny hole at 0.52 times the rate of effusion of Xe atoms under the same conditions of temperature and pressure. What is the molecular formula of arsenic(III) sulfide in the gas phase?
A gas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure conditions. It required 105 s for 1.0 L of the gas to effuse. Under identical experimental conditions it required 31 s for 1.0 L of O2 gas to effuse. Calculate the molar mass of the unknown gas. (Remember that the faster the rate of effusion, the shorter the time required for effusion of 1.0 L; in other words, rate is the amount that diffuses over the time it takes to diffuse.)
(b) List two reasons why the gases deviate from ideal behavior.
