Textbook Question
Which of the following figures represents (a) a pure element, (More than one picture might fit each description.)
440
views

 Verified step by step guidance
Verified step by step guidance


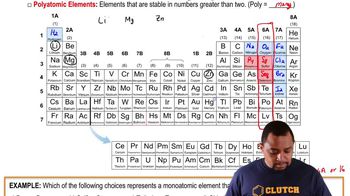
Which of the following figures represents (a) a pure element, (More than one picture might fit each description.)
Which of the following figures represents (c) a pure compound, (More than one picture might fit each description.)
Which of the following figures represents (d) a mixture of an element and a compound? (More than one picture might fit each description.)
Which of the following diagrams represents a chemical change?