Textbook Question
Which of the following figures represents (b) a mixture of two elements, (More than one picture might fit each description.)
193
views

 Verified step by step guidance
Verified step by step guidance

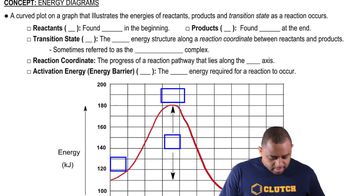
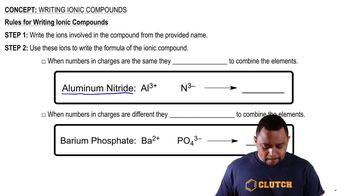
Which of the following figures represents (b) a mixture of two elements, (More than one picture might fit each description.)
Which of the following figures represents (c) a pure compound, (More than one picture might fit each description.)
Which of the following figures represents (d) a mixture of an element and a compound? (More than one picture might fit each description.)