Judge the following statements as true or false. If you believe a statement to be false, provide a corrected version. (i) Compounds always contain at least two different elements.
Ch.1 - Introduction: Matter, Energy, and Measurement

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 1, Problem 94b
In 2009, a team from Northwestern University and Western Washington University reported the preparation of a new 'spongy' material composed of nickel, molybdenum, and sulfur that excels at removing mercury from water. The density of this new material is 0.20 g/cm3, and its surface area is 1242 m2 per gram of material. (b) Calculate the surface area for a 10.0-mg sample of this material.
 Verified step by step guidance
Verified step by step guidance1
Convert the mass of the sample from milligrams to grams by dividing by 1000, since there are 1000 milligrams in a gram.
Use the given surface area per gram of material, which is 1242 m^2/g, to find the total surface area for the sample.
Multiply the mass of the sample in grams by the surface area per gram to calculate the total surface area.
Ensure that the units are consistent and correctly cancel out to leave the final answer in square meters.
Review the calculation to ensure that all steps have been followed correctly and that the units are appropriate.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Surface Area to Mass Ratio
The surface area to mass ratio is a critical concept in material science, particularly for porous materials. It describes how much surface area is available per unit of mass, which can significantly influence the material's reactivity and absorption capabilities. In this case, the high surface area of the spongy material allows for more effective interaction with contaminants like mercury in water.
Recommended video:
Guided course
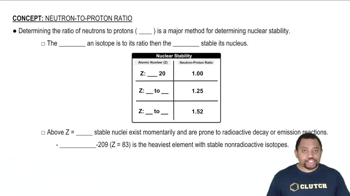
Neutron-Proton Ratio
Unit Conversion
Unit conversion is essential in chemistry for ensuring that measurements are consistent and compatible. In this problem, converting the mass of the sample from milligrams to grams is necessary to align with the surface area measurement given in square meters per gram. Understanding how to convert between different units is fundamental for accurate calculations.
Recommended video:
Guided course

Conversion Factors
Calculation of Surface Area
Calculating the surface area of a sample involves multiplying the surface area per gram by the mass of the sample in grams. This straightforward mathematical operation allows us to determine how much surface area is available for interaction with substances in the environment, which is crucial for applications like water purification.
Recommended video:
Guided course
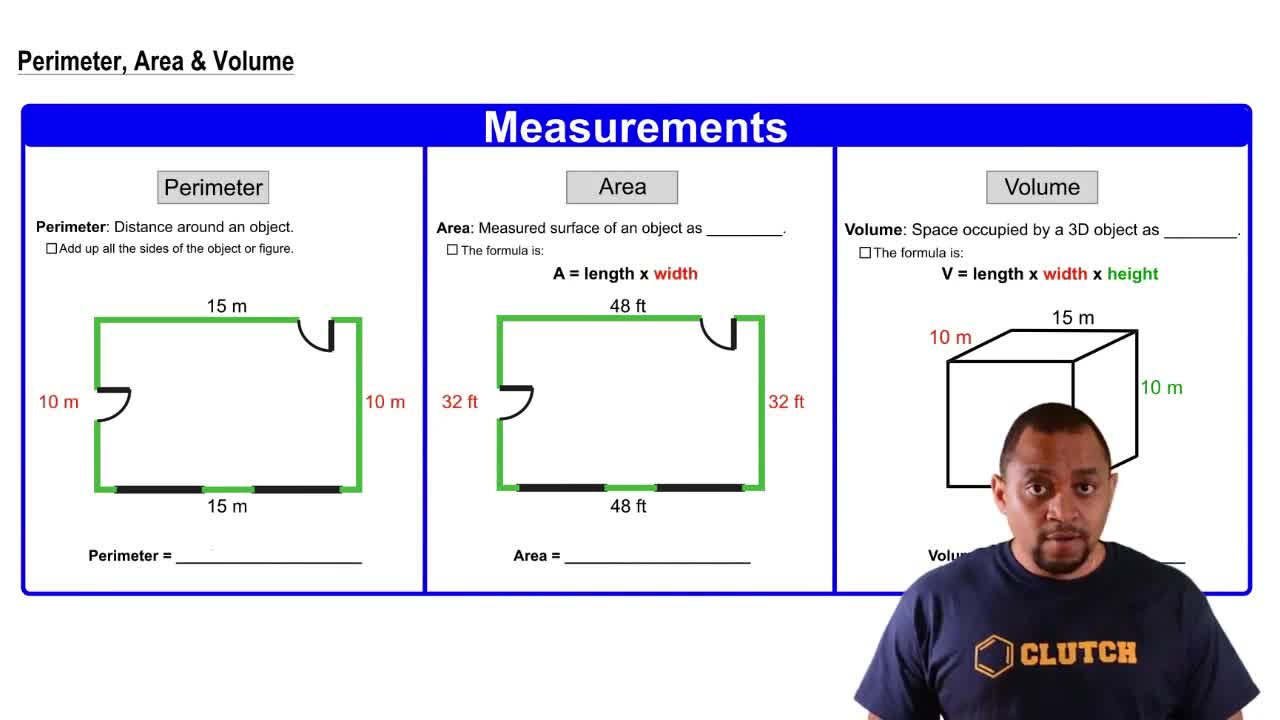
Perimeter, Area, Volume
Related Practice
Textbook Question
32
views
Textbook Question
You are assigned the task of separating a desired granular material with a density of 3.62 g/cm3 from an undesired granular material that has a density of 2.04 g/cm3. You want to do this by shaking the mixture in a liquid in which the heavier material will fall to the bottom and the lighter material will float. A solid will float on any liquid that is more dense. Using an Internet-based source or a handbook of chemistry, find the densities of the following substances: carbon tetrachloride, hexane, benzene, and diiodomethane. Which of these liquids will serve your purpose, assuming no chemical interaction takes place between the liquid and the solids?
Textbook Question
U.S. 1-cent coin (a penny) has a diameter of 19 mm and athickness of 1.5 mm. Assume the coin is made of pure copper,whose density and approximate market price are 8.9 g/cm3and \$2.40 per pound, respectively. Calculate the value ofthe copper in the coin, assuming its thickness is uniform.
Textbook Question
(c) Using the volume of a silver atom and the formula for the volume of a sphere, calculate the radius in angstroms of a silver atom.
