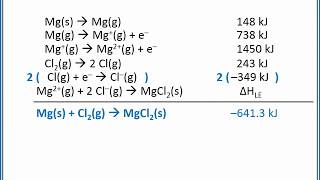11. Bonding & Molecular Structure
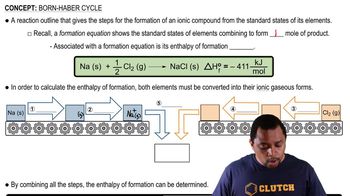
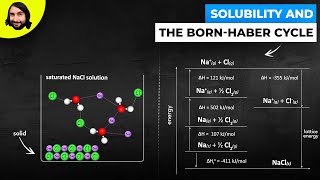
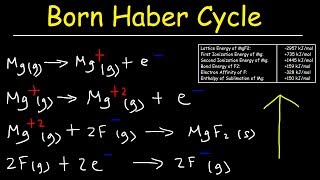
Born Haber Cycle
11. Bonding & Molecular Structure
Born Haber Cycle
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Using the Born-Haber Cycle, demonstrate the formation of cesium chloride, CsCl, and calculate its enthalpy of formation.
- Multiple Choice
Calculate the lattice energy for the following formation equation:
- Open QuestionGiven the following thermodynamic data, calculate the lattice energy of cabr2(s).
- Open QuestionCalculate the lattice formation enthalpy (lattice energy) of the lattice for mgf₂(s), in kj/mol, given the following information:
- Multiple ChoiceCalculate the lattice energy for MgO(s) using a Born-Haber cycle and the following information:Mg(s) → Mg(g) +147.1 kJ/molMg(g) → Mg+(g) + e- +737.8 kJ/molMg+(g) → Mg2+(g) + e- +1451 kJ/mol1/2 O2(g) → O(g) +249.0 kJ/molO(g) + e- → O-(g) -141 kJ/molO-(g) + e- → O2-(g) +844 kJ/molMgO(s) → Mg2+(g) + O2-(g) ?
- Multiple ChoiceWhich of the following steps is NOT part of the Born-Haber cycle for the formation of lithium bromide (LiBr)?