Textbook Question
Predict whether each of the following molecules is polar or nonpolar: (a) IF, (b) CS2, (c) SO3, (d) PCl3, (e) SF6, (f) IF5.

 Verified step by step guidance
Verified step by step guidance
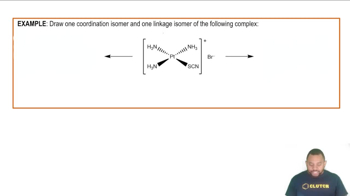
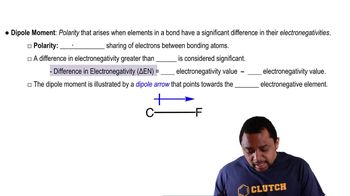
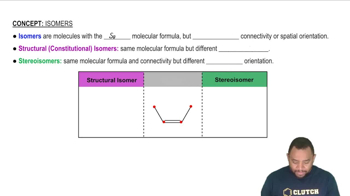
Predict whether each of the following molecules is polar or nonpolar: (a) IF, (b) CS2, (c) SO3, (d) PCl3, (e) SF6, (f) IF5.
Predict whether each of the following molecules is polar or nonpolar: (a) CCl4, (b) NH3, (c) SF4, (d) XeF4, (e) CH3Br, (f) GaH3.
Dichloroethylene (C2H2Cl2) has three forms (isomers), each of which is a different substance. (b) Which of these isomers has a zero dipole moment?
Dihydroxybenzene, C6H6O2, exists in three forms (isomers) called ortho, meta, and para:
Which of these has a nonzero dipole moment?
Draw sketches illustrating the overlap between the following orbitals on two atoms: (a) the 2s orbital on each atom