(b) When subjected to high pressure and heated, polyvinyl chloride converts to diamond. During this transformation which bonds are most likely to break first?

Silicon has the diamond structure with a unit cell edge length of 5.43 Å and eight atoms per unit cell. (b) Suppose you dope that 1 cm3 sample of silicon with 1 ppm of phosphorus that will increase the conductivity by a factor of a million. How many milligrams of phosphorus are required?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Doping in Semiconductors

Conductivity and its Measurement
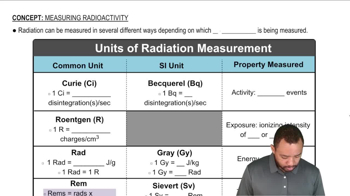
Unit Conversion and Mass Calculation

(c) Employing the values of average bond enthalpy in Table 8.3, estimate the overall enthalpy change for converting PVC to diamond.
Silicon has the diamond structure with a unit cell edge length of 5.43 Å and eight atoms per unit cell. (a) How many silicon atoms are there in 1 cm3 of material?
One method to synthesize ionic solids is by the heating of two reactants at high temperatures. Consider the reaction of FeO with TiO2 to form FeTiO3. Determine the amount of each of the two reactants to prepare 2.500 g FeTiO3, assuming the reaction goes to completion. (a) Write a balanced chemical reaction. (c) Determine the moles of FeTiO3.
One method to synthesize ionic solids is by the heating of two reactants at high temperatures. Consider the reaction of FeO with TiO2 to form FeTiO3. Determine the amount of each of the two reactants to prepare 2.500 g FeTiO3, assuming the reaction goes to completion. (b) Calculate the formula weight of FeTiO3.
One method to synthesize ionic solids is by the heating of two reactants at high temperatures. Consider the reaction of FeO with TiO2 to form FeTiO3. Determine the amount of each of the two reactants to prepare 2.500 g FeTiO3, assuming the reaction goes to completion. (d) Determine moles and mass (g) of FeO required.
