You are on your dream vacation at the beach when a major storm knocks out the power for days. Your cell phone is dead, and you want to make a battery to charge it. You find the following materials in the beach house. blue stone algaecide for pools, which can be used to make a 1.0 M Cu2+ solution alum in the kitchen, which can be used to make a 1.0 M Al3+ solution aluminum foil, copper wire, and bologna, which can be used as a salt bridge. (d) An iPhone requires 5.0 V for charging. Can this battery charge the phone? Explain.

A storm has knocked out power to your beach house, and you would like to build a battery from household items to charge your iPhone. You have the following materials. alum in the kitchen, which can be used to make a 1.0 M Al3+ solution bleach, which is a solution that is approximately a 1.0 M in ClO-aluminum foil, a platinum necklace and bologna, which can be used as a salt bridge (d) An iPhone requires 5.0 V for charging. Can this battery charge the phone? Explain.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Electrochemistry
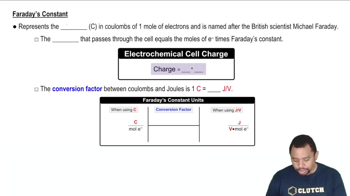
Cell Potential and Voltage

Salt Bridge Functionality
A storm has knocked out power to your beach house, and you would like to build a battery from household items to charge your iPhone. You have the following materials. alum in the kitchen, which can be used to make a 1.0 M Al3+ solution bleach, which is a solution that is approximately a 1.0 M in ClO-aluminum foil, a platinum necklace and bologna, which can be used as a salt bridge (a) What are the half-reactions and overall reaction in the battery?
A storm has knocked out power to your beach house, and you would like to build a battery from household items to charge your iPhone. You have the following materials. alum in the kitchen, which can be used to make a 1.0 M Al3+ solution bleach, which is a solution that is approximately a 1.0 M in ClO-aluminum foil, a platinum necklace and bologna, which can be used as a salt bridge (b) What voltage can be generated?
A mercury battery uses the following electrode half-reactions: (b) Calculate ∆G° (in kilojoules) and K at 25 °C for the cell reaction.
A mercury battery uses the following electrode half-reactions: (c) What is the effect on the cell voltage of a tenfold change in the concentration of KOH in the electrolyte? Explain..
