For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(a) [Pt(NH3)4]2+ (square planar)

 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.128a
Problem 21.128a Verified step by step guidance
Verified step by step guidance
For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(a) [Pt(NH3)4]2+ (square planar)
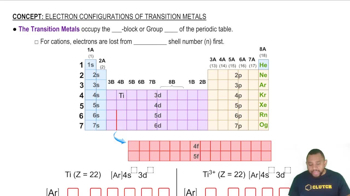
Draw a crystal field energy-level diagram, and predict the number of unpaired electrons for each of the following:
(a) [Mn(H2O)6]2+
Cobalt(III) trifluoroacetylacetonate, Co(tfac)3, is a sixc oordinate, octahedral metal chelate in which three planar, bidentate tfac ligands are attached to a central Co atom:
(a) Draw all possible diastereoisomers and enantiomers of Co(tfac)3.
Tell how many diastereoisomers are possible for each of the following complexes, and draw their structures.
(a) Pt(NH3)3Cl (square planar)
(b) [FeBr2Cl2(en)]-
Assign a systematic name to each of the following ions.
(a) [AuCl4]-
(b) [Fe(CN)6]4-
Two first-series transition metals have three unpaired electrons in complex ions of the type [MCl4]2-.
(a) What are the oxidation state and the identity of M in these complexes?
(b) Draw valence bond orbital diagrams for the two possible ions.
(c) Based on common oxidation states of first-series transition metals (Figure 21.6), which ion is more likely to exist?
<QUESTION REFERENCES FIGURE 21.6>