Multiple Choice
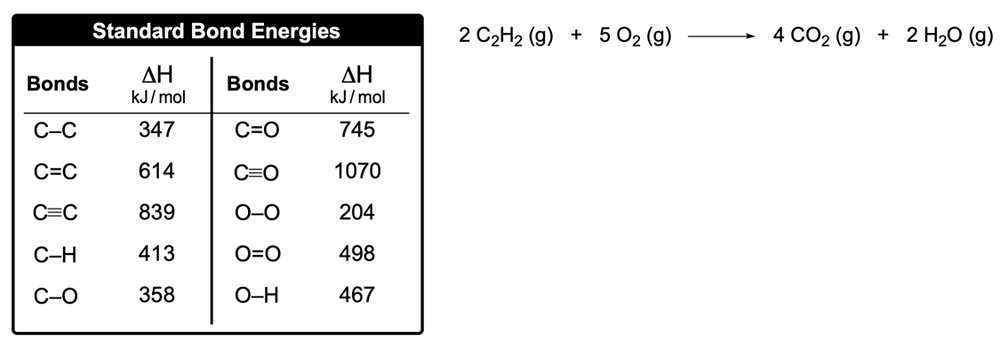
Use the bond energies to determine the approximate enthalpy change for the following reaction: H2(g) + 1/2 O2(g) → H2O(g). Given the bond energies: H-H = 436 kJ/mol, O=O = 498 kJ/mol, and O-H = 463 kJ/mol.
-1074 kJ
2685 kJ
-5020 kJ
-430 kJ
 Verified step by step guidance
Verified step by step guidance