A minimum energy of 7.21⨉10-19 J is required to produce the photoelectric effect in chromium metal. (a) What is the minimum frequency of light needed to remove an electron from chromium?

An energetically excited hydrogen atom has its electron in a 5f subshell. The electron drops down to the 3d subshell, releasing a photon in the process. (b) What wavelength of light is emitted by the process?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Energy Levels and Electron Transitions
Photon Energy and Wavelength Relationship

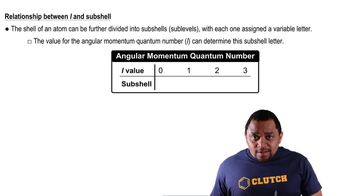
Subshells and Their Energy Levels
A minimum energy of 7.21⨉10-19 J is required to produce the photoelectric effect in chromium metal. (b) Light with a wavelength of 2.50⨉10-7 m falls on a piece of chromium in an evacuated glass tube. What is the minimum de Broglie wavelength of the emitted electrons? (Note that the energy of the incident light must be conserved; that is, the photon's energy must equal the sum of the energy needed to eject the electron plus the kinetic energy of the electron.)
An energetically excited hydrogen atom has its electron in a 5f subshell. The electron drops down to the 3d subshell, releasing a photon in the process. (c) The hydrogen atom now has a single electron in the 3d subshell. What is the energy in kJ/mol required to remove this electron?
Consider the noble gas xenon. (a) Write the electron configuration of xenon using the abbreviation of the previous noble gas.
Consider the noble gas xenon. (c) The energy required to completely remove the outermost electron from the excited xenon atom is 369 kJ/mol, almost identical to that of cesium (376 kJ/mol). Explain.
