An aqueous NaCl solution is made using 102 g of NaCl diluted to a total solution volume of 1.00 L. Calculate the molarity of the solution. (Assume a density of 1.08 g>mL for the solution.)

An aqueous NaCl solution is made using 102 g of NaCl diluted to a total solution volume of 1.00 L. Calculate the mass percent of the solution. (Assume a density of 1.08 g>mL for the solution.)
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Mass Percent

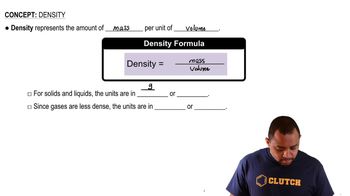
Density
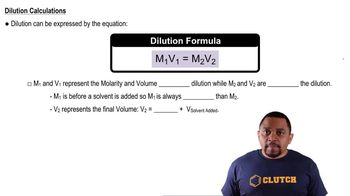
Dilution
An aqueous NaCl solution is made using 102 g of NaCl diluted to a total solution volume of 1.00 L. Calculate the molality of the solution. (Assume a density of 1.08 g>mL for the solution.)
An aqueous KNO3 solution is made using 55.3 g of KNO3 diluted to a total solution volume of 2.00 L. Calculate the molarity of the solution. (Assume a density of 1.05 g>mL for the solution.)
An aqueous KNO3 solution is made using 55.3 g of KNO3 diluted to a total solution volume of 2.00 L. Calculate the molality of the solution. (Assume a density of 1.05 g>mL for the solution.)
An aqueous KNO3 solution is made using 55.3 g of KNO3 diluted to a total solution volume of 2.00 L. Calculate the mass percent of the solution. (Assume a density of 1.05 g>mL for the solution.)
