Scuba divers breathing air at increased pressure can suffer from oxygen toxicity—too much oxygen in their bloodstream— when the partial pressure of oxygen exceeds about 1.4 atm. What happens to the amount of oxygen in a diver's bloodstream when he or she breathes oxygen at elevated pressures? How can this be reversed?
Ch.14 - Solutions

Chapter 14, Problem 57a
An aqueous NaCl solution is made using 102 g of NaCl diluted to a total solution volume of 1.00 L. Calculate the molarity of the solution. (Assume a density of 1.08 g>mL for the solution.)
 Verified step by step guidance
Verified step by step guidance1
Calculate the molar mass of NaCl by adding the atomic masses of sodium (Na) and chlorine (Cl).
Convert the mass of NaCl (102 g) to moles using the molar mass calculated in the previous step.
Determine the volume of the solution in liters, which is given as 1.00 L.
Use the formula for molarity: \( M = \frac{\text{moles of solute}}{\text{liters of solution}} \) to find the molarity of the NaCl solution.
Substitute the moles of NaCl and the volume of the solution into the molarity formula to calculate the molarity.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molarity
Molarity is a measure of concentration defined as the number of moles of solute per liter of solution. It is expressed in units of moles per liter (mol/L). To calculate molarity, one must first determine the number of moles of the solute, which can be found by dividing the mass of the solute by its molar mass, and then divide that by the total volume of the solution in liters.
Recommended video:
Guided course

Molarity
Moles and Molar Mass
A mole is a unit in chemistry that represents 6.022 x 10²³ entities, such as atoms or molecules. The molar mass of a substance is the mass of one mole of that substance, typically expressed in grams per mole (g/mol). For NaCl, the molar mass is approximately 58.44 g/mol, which is essential for converting grams of NaCl into moles for the molarity calculation.
Recommended video:
Guided course

Molar Mass Concept
Density and Volume Conversion
Density is defined as mass per unit volume and is crucial for converting between mass and volume. In this problem, the density of the solution (1.08 g/mL) can be used to confirm the total mass of the solution if needed, but primarily, it helps in understanding how the mass of solute and the volume of the solution relate to each other when calculating molarity.
Recommended video:
Guided course
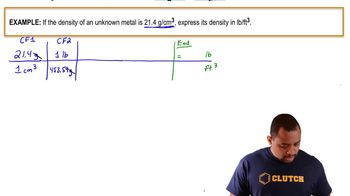
Density Conversion Example
Related Practice
Textbook Question
Textbook Question
An aqueous NaCl solution is made using 102 g of NaCl diluted to a total solution volume of 1.00 L. Calculate the molality of the solution. (Assume a density of 1.08 g>mL for the solution.)
Textbook Question
An aqueous NaCl solution is made using 102 g of NaCl diluted to a total solution volume of 1.00 L. Calculate the mass percent of the solution. (Assume a density of 1.08 g>mL for the solution.)
Textbook Question
An aqueous KNO3 solution is made using 55.3 g of KNO3 diluted to a total solution volume of 2.00 L. Calculate the molarity of the solution. (Assume a density of 1.05 g>mL for the solution.)
