Textbook Question
How many molecules are in each sample? b. 389 g CBr4
1
views

 Verified step by step guidance
Verified step by step guidance

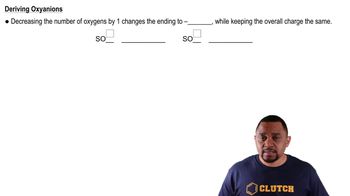
How many molecules are in each sample? b. 389 g CBr4
Write the formula for each molecular compound. a. boron tribromide b. dichlorine monoxide c. xenon tetrafluoride d. carbon tetrabromide
How many atoms are specified by each of these prefixes: mono-, di-, tri-, tetra-, penta-, hexa-?
Determine the number of each type of atom in each formula. a. Mg3(PO4)2 b. BaCl2 c. Fe(NO2)2 d. Ca(OH)2