Textbook Question
Write the formula for each molecular compound. a. boron tribromide b. dichlorine monoxide c. xenon tetrafluoride d. carbon tetrabromide

 Verified step by step guidance
Verified step by step guidance

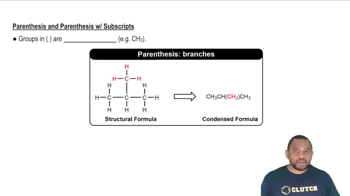

Write the formula for each molecular compound. a. boron tribromide b. dichlorine monoxide c. xenon tetrafluoride d. carbon tetrabromide
How many atoms are specified by each of these prefixes: mono-, di-, tri-, tetra-, penta-, hexa-?
Determine the number of each type of atom in each formula. a. Ca(NO2)2 d. Mg(HCO3)2
Determine the number of each type of atom in each formula. b. CuSO4 d. Mg(HCO3)2
Determine the number of each type of atom in each formula. c. Al(NO3)3