Calculate the number of moles of solute present in each of the following solutions: (a) 255 mL of 1.50 M HNO3(aq),
Ch.13 - Properties of Solutions

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 13, Problem 53c
Describe how you would prepare each of the following aqueous solutions, starting with solid KBr: (c) 1.85 L of a solution that is 12.0% KBr by mass (the density of the solution is 1.10 g/mL)
 Verified step by step guidance
Verified step by step guidance1
Calculate the total mass of the solution using the volume and density. Use the formula: \( \text{mass} = \text{volume} \times \text{density} \). Convert the volume from liters to milliliters first, since the density is given in g/mL.
Determine the mass of KBr required for the solution. Since the solution is 12.0% KBr by mass, use the formula: \( \text{mass of KBr} = \text{total mass of solution} \times \frac{12.0}{100} \).
Convert the mass of KBr from grams to moles if needed, using the molar mass of KBr. The molar mass of KBr can be calculated by adding the atomic masses of potassium (K) and bromine (Br).
Weigh the calculated mass of solid KBr using a balance.
Dissolve the weighed KBr in a container with water, ensuring the final volume of the solution is 1.85 L. Stir the solution until the KBr is completely dissolved.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Mass Percent Concentration
Mass percent concentration is a way to express the concentration of a solute in a solution, calculated as the mass of the solute divided by the total mass of the solution, multiplied by 100. In this case, a 12.0% KBr solution means that there are 12 grams of KBr in every 100 grams of the solution. Understanding this concept is essential for determining how much KBr is needed to achieve the desired concentration.
Recommended video:
Guided course

Mass Percent Calculation
Density of Solutions
Density is defined as mass per unit volume and is crucial for converting between mass and volume in solutions. The density of the solution provided (1.10 g/mL) allows us to calculate the total mass of the solution based on its volume. This relationship is vital for determining how much KBr to add to reach the specified mass percent concentration in the final solution.
Recommended video:
Guided course
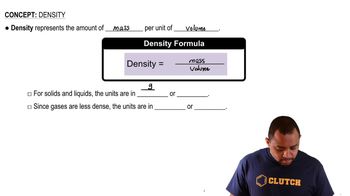
Density Concepts
Solution Preparation
Preparing a solution involves accurately measuring and mixing the solute with a solvent to achieve a desired concentration. In this scenario, one must first calculate the total mass of the solution needed using its volume and density, then determine the mass of KBr required based on the mass percent concentration. Finally, the solid KBr is dissolved in an appropriate amount of solvent to create the final solution.
Recommended video:
Guided course
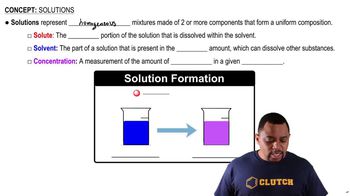
Solution Components
Related Practice
Textbook Question
Textbook Question
Describe how you would prepare each of the following aqueous solutions, starting with solid KBr: (b) 125 g of 0.180 m KBr,
Textbook Question
Describe how you would prepare each of the following aqueous solutions, starting with solid KBr: (d) a 0.150 M solution of KBr that contains just enough KBr to precipitate 16.0 g of AgBr from a solution containing 0.480 mol of AgNO3.
Textbook Question
Describe how you would prepare each of the following aqueous solutions: (a) 1.50 L of 0.110 M 1NH422SO4 solution, starting with solid 1NH422SO4;
Textbook Question
Describe how you would prepare each of the following aqueous solutions: (c) 1.20 L of a solution that is 15.0% Pb(NO3)2 by mass (the density of the solution is 1.16 g/mL), starting with solid solute;
