The probe of the pH meter shown here is sitting in a beaker that contains a clear liquid. You are told the liquid is pure water, a solution of HCl(aq), or a solution of KOH(aq). (b) If the liquid is one of the solutions, what is its molarity?

The following diagrams represent aqueous solutions of three acids, HX, HY, and HZ. The water molecules have been omitted for clarity, and the hydrated proton is represented as H+ rather than H3O+.(b) Which acid would have the smallest aciddissociation constant, Ka?

 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Acid Dissociation Constant (Ka)
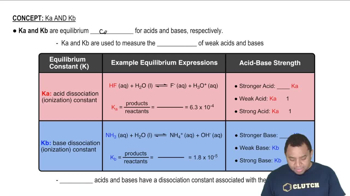
Strength of Acids
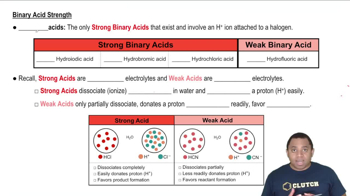
Molecular Structure and Electronegativity

The probe of the pH meter shown here is sitting in a beaker that contains a clear liquid. (c) Why is the temperature given on the pH meter?
The following diagrams represent aqueous solutions of three acids, HX, HY, and HZ. The water molecules have been omitted for clarity, and the hydrated proton is represented as H+ rather than H3O+. (a) Which of the acids is a strong acid? Explain.
The following diagrams represent aqueous solutions of three acids, HX, HY, and HZ. The water molecules have been omitted for clarity, and the hydrated proton is represented as H+ rather than H3O+.(c) Which solution would have the highest pH?
Each of the three molecules shown here contains an OH group, but one molecule acts as a base, one as an acid, and the third is neither acid nor base. (c) Which one is neither acidic nor basic?
Phenylephrine, an organic substance with molecular formula C9H13NO2, is used as a nasal decongenstant in over-thecounter medications. The molecular structure of phenylephrine is shown below using the usual shortcut organic structure. (a) Would you expect a solution of phenylephrine to be acidic, neutral, or basic?
