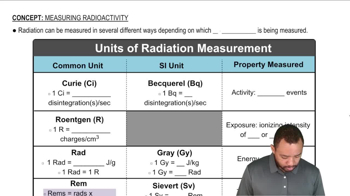
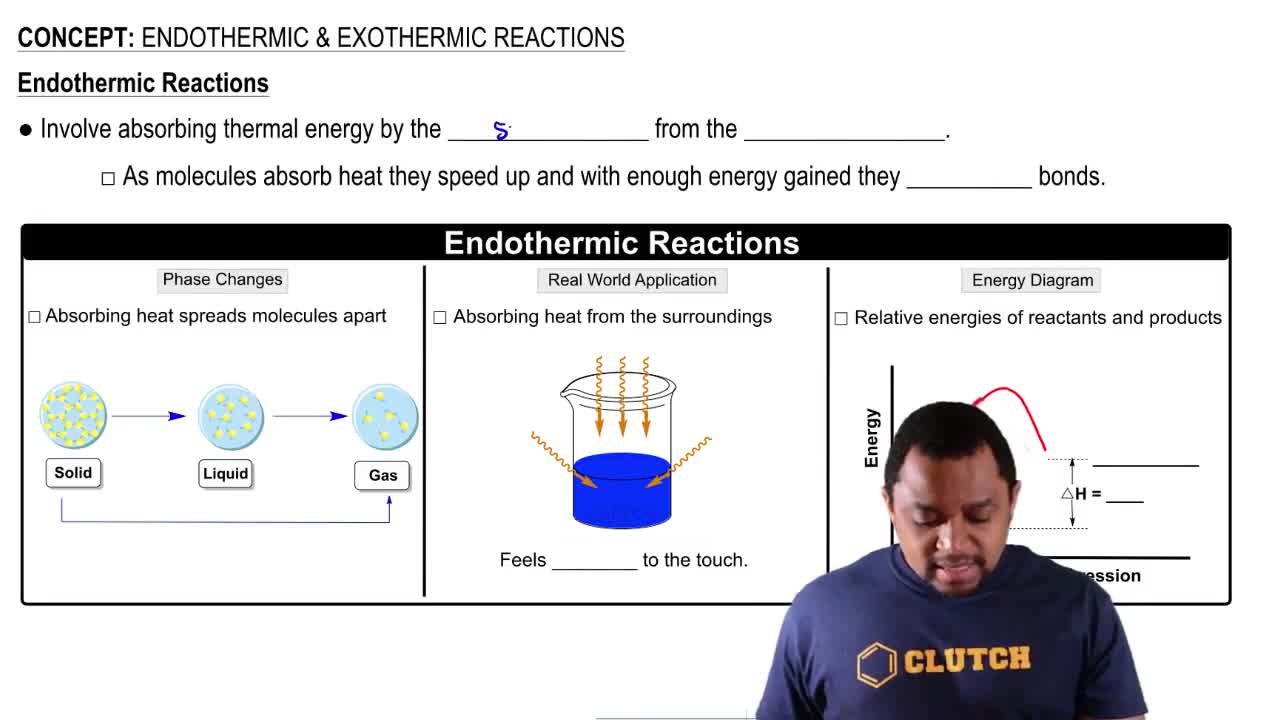
Textbook Question
Fraud in science is rare but does happen occasionally. In 1999, the creation of three superheavy elements (one new) was claimed when 208Pb was bombarded with 86Kr. The claim was subsequently found to be fraudulent and was with-drawn. Identify the isotopes X, Y, and Z that were claimed.