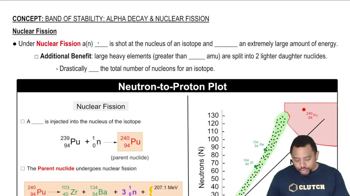
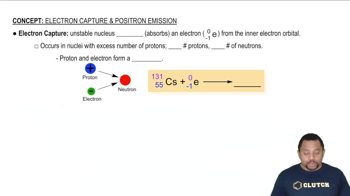
Textbook Question
One of the new superheavy elements added to the periodic table in 2016 was synthesized when a beam of 48Ca ions was directed at a target of 243Am. (a) Write a balanced nuclear equation for the formation of this element. (b) What isotope is formed after the nuclide formed in the nuclear transmutation reaction in part (a) emits one alpha particle?(c) How many alpha particles were emitted to reach the isotope 268Db, the final decay product?