Spinach contains a lot of iron but is not a good source of dietary iron because nearly all the iron is tied up in the oxalate complex [Fe(C2O4)3]3-.
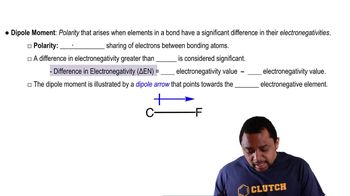
(c) Draw a crystal field energy-level diagram for [Fe(C2O4)3]3-, and predict the number of unpaired electrons. (C2O42- is a weak-field bidentate ligand.)