Equilibrium in Aqueous Solutions
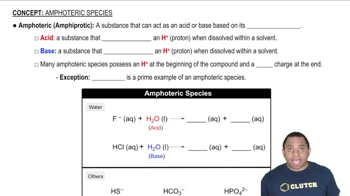
In aqueous solutions, chemical species exist in dynamic equilibrium, meaning their concentrations can change based on reactions occurring in the solution. Understanding this equilibrium is essential for predicting how species like H2S, HS-, and S2- will behave as acids or bases, as well as how they interact with water and other ions present in the solution.

 Verified step by step guidance
Verified step by step guidance