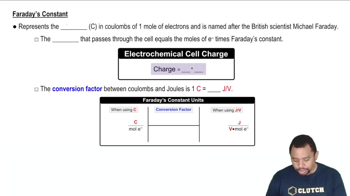
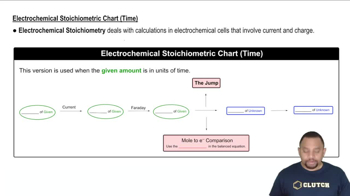
Textbook Question
Aluminum, titanium, and several other metals can be col-ored by an electrochemical process called anodizing. Anodizing oxidizes a metal anode to yield a porous metal oxide coating that can incorporate dye molecules to give brilliant colors. In the oxidation of aluminum, for instance, the electrode reactions are The thickness of the aluminum oxide coating that forms on the anode can be controlled by varying the current flow during the electrolysis. How many minutes are required to produce a 0.0100-mm thick coating of Al2O3 (density 3.97 g/cm^3) on a square piece of aluminum metal 10.0 cm on an edge if the current passed through the piece is 0.600 A?
8
views