Textbook Question
In order to charge a lead storage battery (Section 19.10) 500.0 g of PbSO4(s) must be converted into PbO2(s) and Pb(s). (c) If a current of 500 A is used, how long will it take?

 Verified step by step guidance
Verified step by step guidance


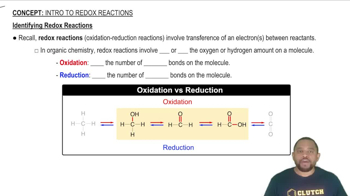
In order to charge a lead storage battery (Section 19.10) 500.0 g of PbSO4(s) must be converted into PbO2(s) and Pb(s). (c) If a current of 500 A is used, how long will it take?
When the nickel–zinc battery, used in digital cameras, is recharged, the following cell reaction occurs: (a) How many grams of zinc are formed when 3.35 x 10-2 g of Ni(OH)2 are consumed?
Chlorine can be prepared in the laboratory by the reaction of hydrochloric acid and potassium permanganate. (a) Use data in Appendix D to write a balanced equation for the reaction. The reduction product is Mn2+.