(c) By what means can the internal energy of a closed system increase?

Calculate ΔE and determine whether the process is endothermic or exothermic for the following cases: (b) A system releases 66.1 kJ of heat to its surroundings while the surroundings do 44.0 kJ of work on the system.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
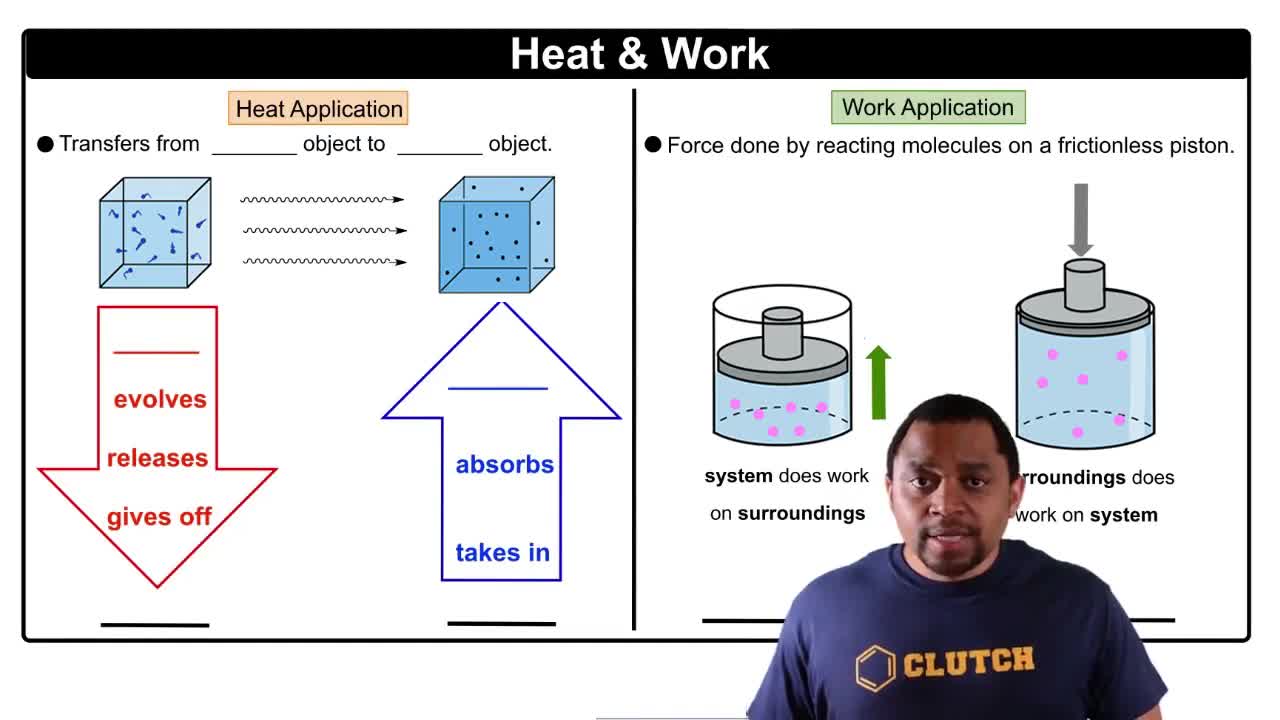
Key Concepts
First Law of Thermodynamics

Endothermic and Exothermic Processes

Work and Heat in Thermodynamics
Calculate ΔE and determine whether the process is endothermic or exothermic for the following cases: (a) q = 0.763 kJ and w = -840 J.
For the following processes, calculate the change in internal energy of the system and determine whether the process is endothermic or exothermic: (a) A balloon is cooled by removing 0.655 kJ of heat. It shrinks on cooling, and the atmosphere does 382 J of work on the balloon. (b) A 100.0-g bar of gold is heated from 25 °C to 50 °C during which it absorbs 322 J of heat. Assume the volume of the gold bar remains constant.
A gas is confined to a cylinder fitted with a piston and an electrical heater, as shown here:
Suppose that current is supplied to the heater so that 100 J of energy is added. Consider two different situations. In case (1) the piston is allowed to move as the energy is added. In case (2) the piston is fixed so that it cannot move. (a) In which case does the gas have the higher temperature after addition of the electrical energy?
A gas is confined to a cylinder fitted with a piston and an electrical heater, as shown here:
Suppose that current is supplied to the heater so that 100 J of energy is added. Consider two different situations. In case (1) the piston is allowed to move as the energy is added. In case (2) the piston is fixed so that it cannot move. (b) Identify the sign (positive, negative, or zero) of q and w in each case?
