(a) The EPA threshold for acceptable levels of lead ions in water is 615 ppb. What is the molarity of an aqueous solution with a concentration of 15 ppb?

The estimated average concentration of NO2 in air in the United States in 2006 was 0.016 ppm. (a) Calculate the partial pressure of the NO2 in a sample of this air when the atmospheric pressure is 755 torr (99.1 kPa).
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Partial Pressure

Concentration Units
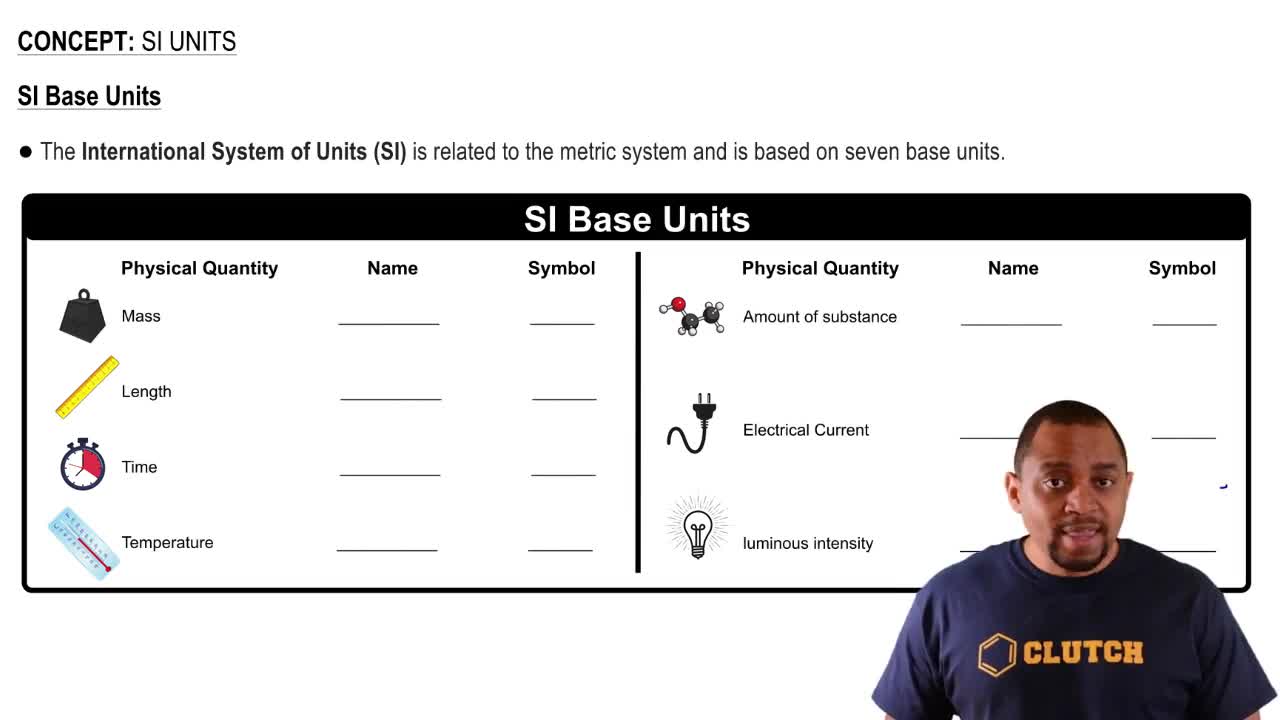
Gas Laws
(b) Concentrations of lead in the bloodstream are often quoted in units of μg/dL. Averaged over the entire country, the mean concentration of lead in the blood was measured to be 1.6 μg/dL in 2008. Express this concentration in ppb.
In 1986 an electrical power plant in Taylorsville, Georgia, burned 8,376,726 tons of coal, a national record at that time. (a) Assuming that the coal was 83% carbon and 2.5% sulfur and that combustion was complete, calculate the number of tons of carbon dioxide and sulfur dioxide produced by the plant during the year.
In 1986 an electrical power plant in Taylorsville, Georgia, burned 8,376,726 tons of coal, a national record at that time. (b) If 55% of the SO2 could be removed by reaction with powdered CaO to form CaSO3, how many tons of CaSO3 would be produced?
The water supply for a midwestern city contains the following impurities: coarse sand, finely divided particulates, nitrate ions, trihalomethanes, dissolved phosphorus in the form of phosphates, potentially harmful bacterial strains, dissolved organic substances. Which of the following processes or agents, if any, is effective in removing each of these impurities: coarse sand filtration, activated carbon filtration, aeration, ozonization, precipitation with aluminum hydroxide?
