(a) Which kind of intermolecular attractive force is shown in each case here? (b) Predict which of the four interactions is the weakest. [Section 11.2]
Ch.11 - Liquids and Intermolecular Forces

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 11, Problem 3
a. Which of the molecules shown here can form dipole–dipole interactions with other molecules of the same type?
b. Which are capable of forming hydrogen bonds with other molecules of the same type? [Section 11.2]
 Verified step by step guidance
Verified step by step guidance1
Identify the molecular structures of the given molecules and determine if they have polar bonds by checking the electronegativity differences between bonded atoms.
Assess the overall molecular polarity by considering the molecular geometry and the vector sum of the bond dipoles. Molecules with a net dipole moment can engage in dipole-dipole interactions.
For hydrogen bonding, check if the molecule contains hydrogen directly bonded to highly electronegative atoms such as nitrogen, oxygen, or fluorine, which is a prerequisite for hydrogen bonding.
Evaluate the ability of these electronegative atoms to serve as hydrogen bond acceptors by checking if they have lone pairs of electrons, which can interact with hydrogen atoms of other molecules.
Summarize which molecules meet the criteria for dipole-dipole interactions and hydrogen bonding based on the analysis of their molecular structure and bonding.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Dipole-Dipole Interactions
Dipole-dipole interactions occur between polar molecules, where the positive end of one molecule is attracted to the negative end of another. These interactions arise due to the uneven distribution of electron density, leading to partial positive and negative charges. Molecules with permanent dipoles can engage in these interactions, which are generally stronger than London dispersion forces but weaker than hydrogen bonds.
Recommended video:
Guided course
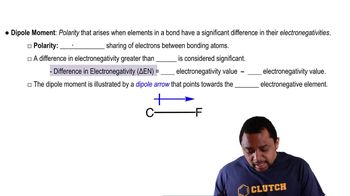
Dipole Moment
Hydrogen Bonding
Hydrogen bonding is a specific type of dipole-dipole interaction that occurs when hydrogen is covalently bonded to highly electronegative atoms like nitrogen, oxygen, or fluorine. This bond creates a significant dipole, allowing hydrogen to interact strongly with lone pairs of electrons on nearby electronegative atoms. Hydrogen bonds are crucial in determining the properties of substances like water and biological molecules such as DNA.
Recommended video:
Guided course

Hydrogenation Reactions
Polarity of Molecules
The polarity of a molecule is determined by its shape and the electronegativity of its constituent atoms. A molecule is polar if it has a net dipole moment, meaning that the distribution of electron density is uneven, leading to distinct positive and negative regions. Understanding polarity is essential for predicting the types of intermolecular forces that can occur, including dipole-dipole interactions and hydrogen bonding.
Recommended video:
Guided course

Molecular Polarity
Related Practice
Textbook Question
4
views
Textbook Question
If 42.0 kJ of heat is added to a 32.0-g sample of liquid meth-ane under 1 atm of pressure at a temperature of -170°C, what are the final state and temperature of the methane once the system equilibrates? Assume no heat is lost to the surroundings. The normal boiling point of methane is -161.5 °C. The specific heats of liquid and gaseous methane are 3.48 and 2.22 J/g-K, respectively. [Section 11.4]
Textbook Question
Using this graph of CS2 data, determine (a) the approximate vapor pressure of CS2 at 30°C,
Textbook Question
The molecules
have the same molecular formula (C3H8O) but different chemical structures. (b) Which molecule do you expect to have a larger dipole moment? [Sections 11.2 and 11.5]
4
views
