a. Which of the molecules shown here can form dipole–dipole interactions with other molecules of the same type?
b. Which are capable of forming hydrogen bonds with other molecules of the same type? [Section 11.2]

 Verified step by step guidance
Verified step by step guidance
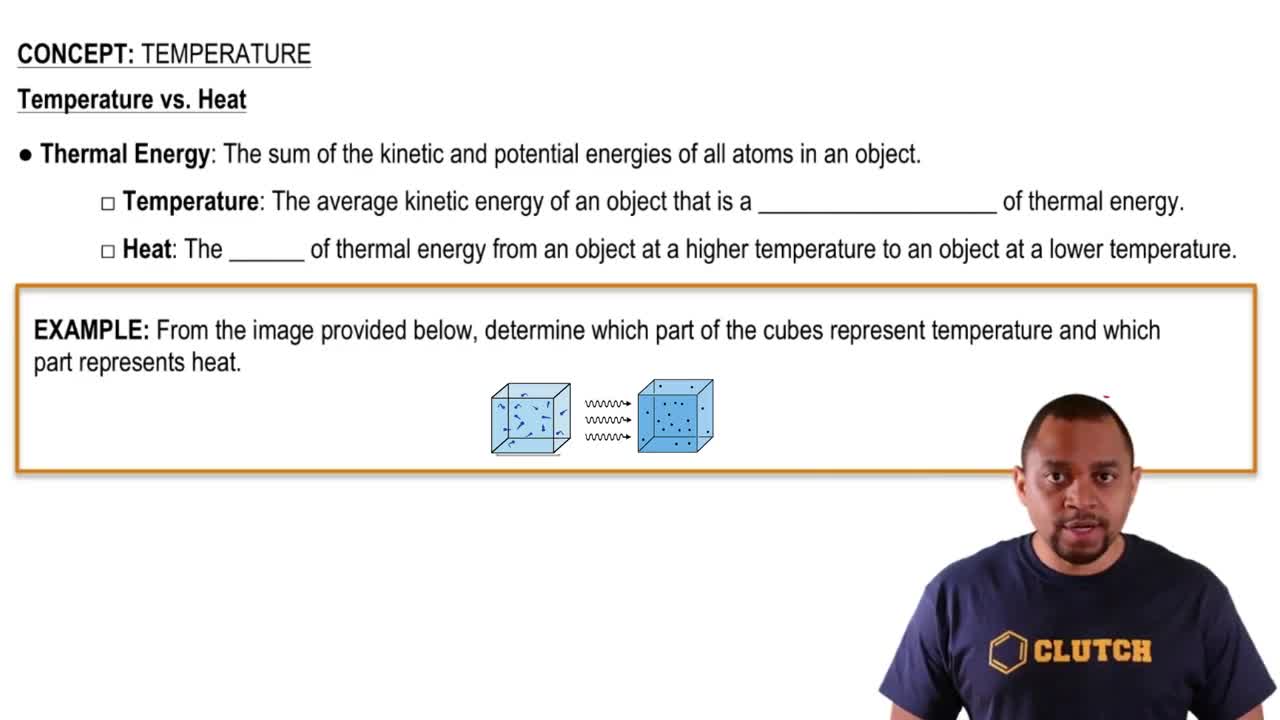
a. Which of the molecules shown here can form dipole–dipole interactions with other molecules of the same type?
b. Which are capable of forming hydrogen bonds with other molecules of the same type? [Section 11.2]
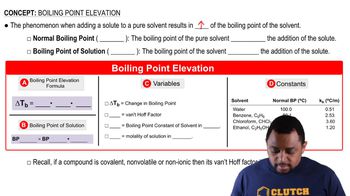
(a) Which kind of intermolecular attractive force is shown in each case here? (b) Predict which of the four interactions is the weakest. [Section 11.2]
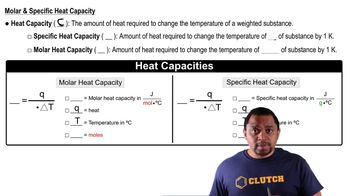
Using this graph of CS2 data, determine (a) the approximate vapor pressure of CS2 at 30°C,
The molecules
have the same molecular formula (C3H8O) but different chemical structures. (b) Which molecule do you expect to have a larger dipole moment? [Sections 11.2 and 11.5]
The phase diagram of a hypothetical substance is
(b) What is the physical state of the substance under the following conditions? (i) T = 150 K, P = 0.2 atm; (ii) T = 100 K, P = 0.8 atm; (iii) T = 300K, P = 1.0atm. [Section 11.6]