At room temperature, Si is a solid, CCl4 is a liquid, and Ar is a gas. List these substances in order of (a) increasing intermolecular energy of attraction

At standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively. (d) Are the molar volumes in the solid state as similar as they are in the gaseous state?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
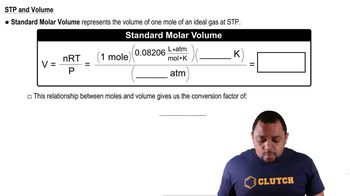
Key Concepts
Molar Volume
States of Matter
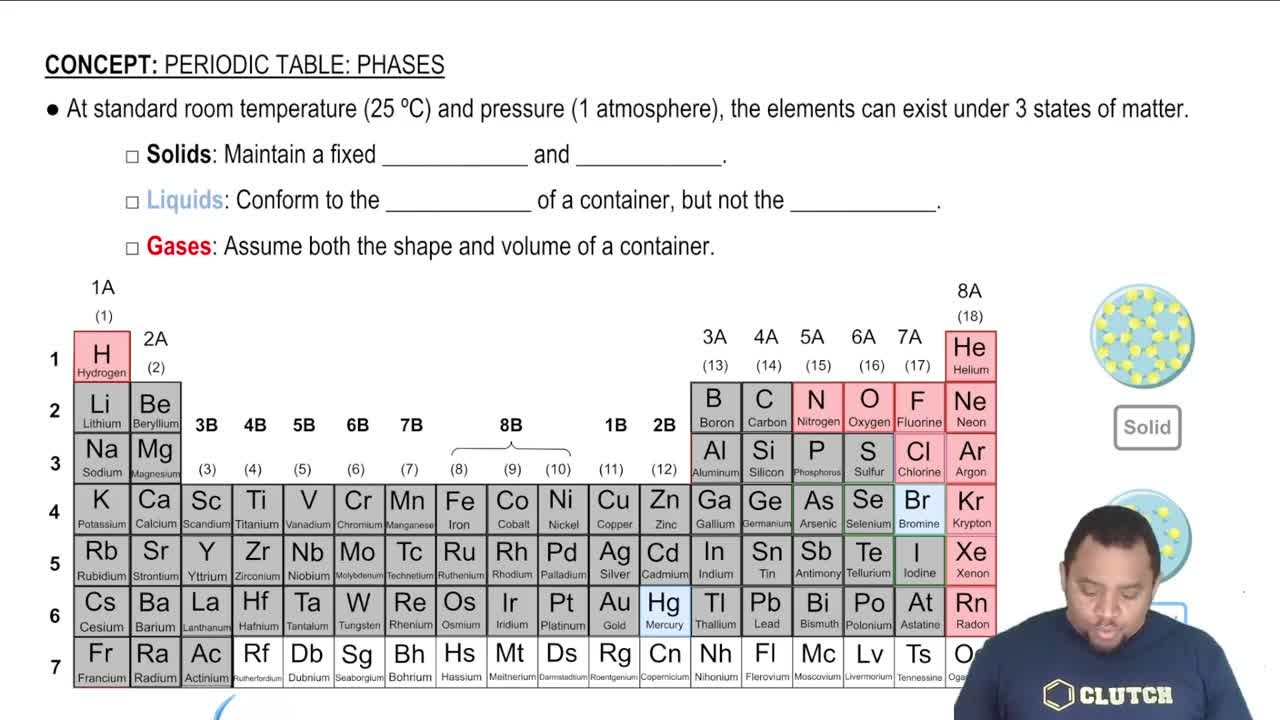
Comparative Analysis of Gases and Solids

At standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively (b) On cooling to 160 K, both substances form crystalline solids. Do you expect the molar volumes to decrease or increase on cooling the gases to 160 K?
At standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively. (c) The densities of crystalline Cl2 and NH3 at 160 K are 2.02 and 0.84 g/cm3, respectively. Calculate their molar volumes.
Benzoic acid, C6H5COOH, melts at 122 °C. The density in the liquid state at 130 °C is 1.08 g/cm3. The density of solid benzoic acid at 15 °C is 1.266 g/cm3. (b) If you converted a cubic centimeter of liquid benzoic acid into a solid, would the solid take up more, or less, volume than the original cubic centimeter of liquid?
(a) Which type of intermolecular attractive force operates between all molecules?
(b) Which type of intermolecular attractive force operates only between polar molecules?
