Consider the reaction: NO2(g) → NO(g) + 1/2 O2( g) The tabulated data were collected for the concentration of NO2 as a function of time: b. What is the rate of formation of O2 between 50 and 60 s?
Ch.14 - Chemical Kinetics

Chapter 14, Problem 33a(iii)
Consider the reaction: H2(g) + Br2(g) → 2 HBr(g) The graph shows the concentration of Br2 as a function of time. a. Use the graph to calculate each quantity: (iii) the instantaneous rate of formation of HBr at 50 s
 Verified step by step guidance
Verified step by step guidance1
Identify the relationship between the rate of disappearance of Br2 and the rate of formation of HBr from the balanced chemical equation: . The stoichiometry shows that 1 mole of Br2 produces 2 moles of HBr.
Determine the instantaneous rate of disappearance of Br2 at 50 seconds by finding the slope of the tangent line to the concentration vs. time graph at that point. This slope represents .
Calculate the instantaneous rate of formation of HBr using the stoichiometric relationship: .
Express the rate of formation of HBr as , ensuring the rate is positive since it is a formation rate.
Summarize the result as the instantaneous rate of formation of HBr at 50 s, based on the slope obtained from the graph and the stoichiometric factor of 2.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Reaction Rate and Instantaneous Rate
The reaction rate measures how fast reactants are consumed or products are formed. The instantaneous rate is the rate at a specific moment, found by calculating the slope of the concentration vs. time graph at that point, often using the tangent line.
Recommended video:
Guided course
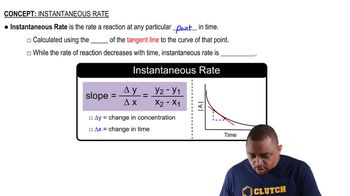
Instantaneous Rate
Stoichiometry and Rate Relationships
Stoichiometry relates the amounts of reactants and products in a chemical reaction. For the reaction H2 + Br2 → 2 HBr, the rate of formation of HBr is twice the rate of consumption of Br2, so rates must be adjusted according to their coefficients.
Recommended video:
Guided course
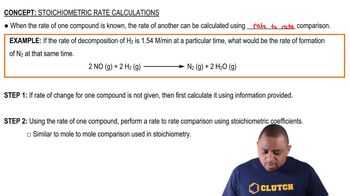
Stoichiometric Rate Calculations
Interpreting Concentration-Time Graphs
Concentration-time graphs show how reactant or product concentrations change over time. The slope of the curve at a given time gives the rate of change of concentration, which can be used to find instantaneous rates for reactants or products.
Recommended video:
Guided course

Intepreting the Band of Stability
Related Practice
Textbook Question
Textbook Question
Consider the reaction: H2(g) + Br2(g) → 2 HBr(g) The graph shows the concentration of Br2 as a function of time.
a. Use the graph to calculate each quantity: (i) the average rate of the reaction between 0 and 25 s
Textbook Question
Consider the reaction: H2( g) + Br2( g) → 2 HBr( g) The graph shows the concentration of Br2 as a function of time.
b. Make a rough sketch of a curve representing the concentration of HBr as a function of time. Assume that the initial concentration of HBr is zero
Textbook Question
Consider the reaction: 2 H2O2(aq) → 2 H2O(l ) + O2( g) The graph shows the concentration of H2O2 as a function of time.
Use the graph to calculate each quantity: c. the instantaneous rate of formation of O2 at 50 s
