Open Question
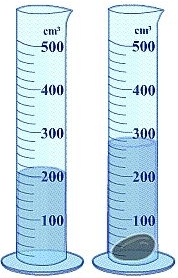
Determine the density of an object that has a mass of 149.8 g and displaces 12.1 mL of water when placed in a graduated cylinder.
 Verified step by step guidance
Verified step by step guidance
 3:01m
3:01mMaster Density via Water Displacement with a bite sized video explanation from Jules
Start learning
