The hydrogen atom can absorb light of wavelength 1094 nm. (b) Determine the final value of n associated with this absorption.
Ch.6 - Electronic Structure of Atoms

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 6, Problem 47a,b
Use the de Broglie relationship to determine the wavelengths of the following objects: (a) an 85-kg person skiing at 50 km/hr (b) a 10.0-g bullet fired at 250 m/s
 Verified step by step guidance
Verified step by step guidance1
Convert the speed from km/hr to m/s. Use the conversion factor: 1 km/hr = 0.27778 m/s.
Use the de Broglie wavelength formula: \( \lambda = \frac{h}{mv} \), where \( \lambda \) is the wavelength, \( h \) is Planck's constant \( 6.626 \times 10^{-34} \text{ m}^2 \text{ kg/s} \), \( m \) is the mass in kg, and \( v \) is the velocity in m/s.
Substitute the mass of the person (85 kg) and the converted velocity into the de Broglie equation.
Calculate the de Broglie wavelength using the substituted values.
Interpret the result to understand the significance of the wavelength in the context of macroscopic objects.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
6mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
de Broglie Wavelength
The de Broglie wavelength is a concept in quantum mechanics that relates the wavelength of a particle to its momentum. It is given by the formula λ = h/p, where λ is the wavelength, h is Planck's constant (6.626 x 10^-34 Js), and p is the momentum of the object. This relationship implies that all matter exhibits wave-like properties, with the wavelength inversely proportional to the momentum.
Recommended video:
Guided course
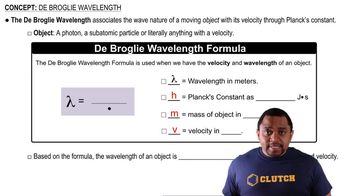
De Broglie Wavelength Formula
Momentum
Momentum is a physical quantity defined as the product of an object's mass and its velocity, expressed as p = mv. In the context of the de Broglie relationship, momentum is crucial because it determines the wavelength of the object. For an object with a significant mass, like an 85-kg person, the momentum will be substantial, leading to a very short wavelength that is typically imperceptible in everyday life.
Recommended video:
Guided course

Angular Momentum Quantum Number
Units of Measurement
Understanding units of measurement is essential for correctly applying the de Broglie relationship. In this case, the mass should be in kilograms (kg) and the velocity in meters per second (m/s) to ensure consistency with the SI units used in Planck's constant. Converting the skiing speed from kilometers per hour to meters per second is necessary for accurate calculations of the wavelength.
Recommended video:
Guided course
Units of Radiation Measurement
Related Practice
Textbook Question
Textbook Question
Order the following transitions in the hydrogen atom from smallest to largest frequency of light absorbed: n = 3 to n = 7, n = 4 to n = 8, n = 2 to n = 5, and n = 1 to n = 3.
1
views
Textbook Question
Write the electron configurations for the following ions, anddetermine which have noble-gas configurations:(a) Ti2+(b) Br-(c) Mg2+(d) Po2-(e) Pt2+(f) V3+
Textbook Question
Use the de Broglie relationship to determine the wavelengths of the following objects: (c) a lithium atom moving at 2.5 × 105 m/s (d) an ozone (O3) molecule in the upper atmosphere moving at 550 m/s.
Textbook Question
Among the elementary subatomic particles of physics is the muon, which decays within a few microseconds after formation. The muon has a rest mass 206.8 times that of an electron. Calculate the de Broglie wavelength associated with a muon traveling at 8.85 * 105 cm/s.
Textbook Question
Neutron diffraction is an important technique for determining the structures of molecules. Calculate the velocity of a neutron needed to achieve a wavelength of 125 pm. (Refer to the inside cover for the mass of the neutron.)
