(c) What is meant by the term standard enthalpy of formation?
Ch.5 - Thermochemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 5, Problem 72a
Write balanced equations that describe the formation of the following compounds from elements in their standard states, and then look up the standard enthalpy of formation for each substance in Appendix C: (a) NH4NO3(s)
 Verified step by step guidance
Verified step by step guidance1
Identify the elements in their standard states that are needed to form NH_4NO_3(s). These are nitrogen (N_2), hydrogen (H_2), and oxygen (O_2).
Write the balanced chemical equation for the formation of NH_4NO_3(s) from its elements: \[ \frac{1}{2}N_2(g) + \frac{3}{2}H_2(g) + \frac{3}{2}O_2(g) \rightarrow NH_4NO_3(s) \]
Ensure that the equation is balanced in terms of both atoms and charge. In this case, the equation is balanced as written.
Look up the standard enthalpy of formation (\( \Delta H_f^\circ \)) for NH_4NO_3(s) in Appendix C or a reliable chemistry data source.
The standard enthalpy of formation is the change in enthalpy when one mole of a compound is formed from its elements in their standard states under standard conditions (298 K and 1 atm).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balanced Chemical Equations
A balanced chemical equation represents a chemical reaction where the number of atoms for each element is the same on both sides of the equation. This is crucial for obeying the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. Balancing equations involves adjusting coefficients to ensure that the total number of each type of atom is equal, reflecting the actual stoichiometry of the reaction.
Recommended video:
Guided course

Balancing Chemical Equations
Standard States of Elements
The standard state of an element refers to its most stable form at a specified temperature (usually 25°C) and pressure (1 atm). For example, the standard state of nitrogen is N2 gas, while that of carbon is graphite. Understanding standard states is essential for accurately writing formation reactions, as the enthalpy of formation is calculated based on elements in their standard states.
Recommended video:
Guided course
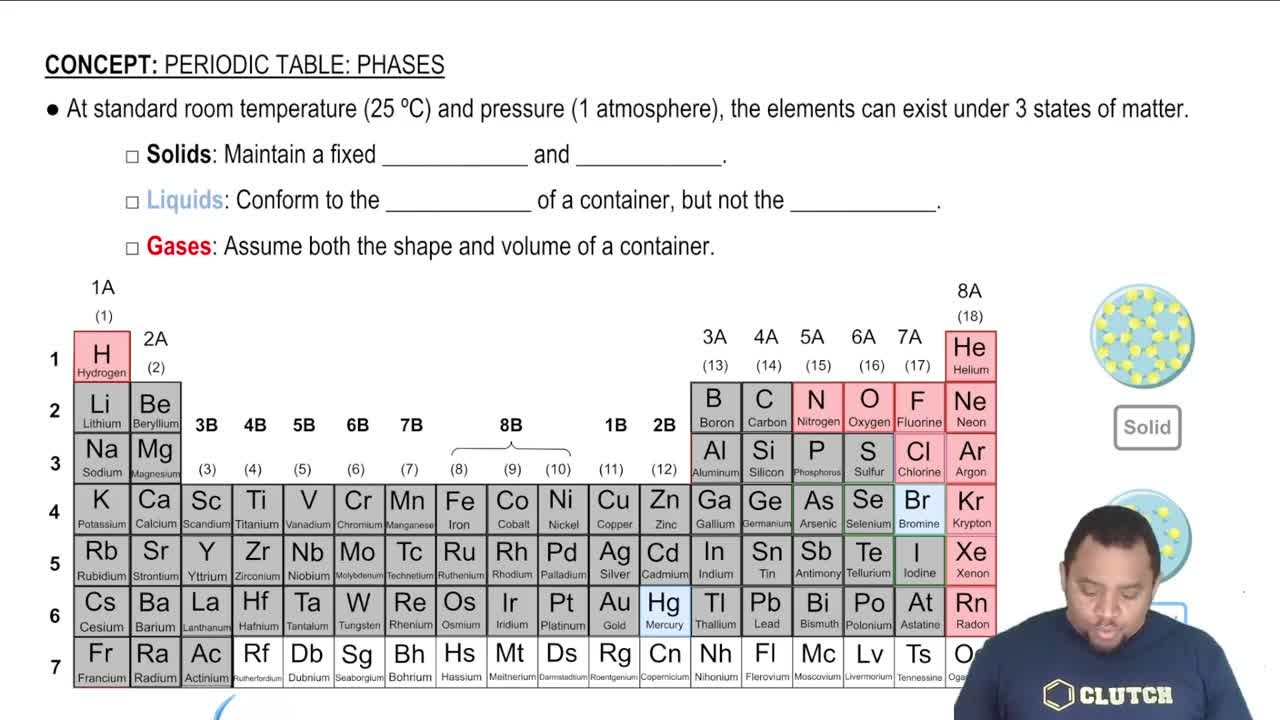
Element States of Matter
Standard Enthalpy of Formation
The standard enthalpy of formation (ΔHf°) is the change in enthalpy when one mole of a compound is formed from its elements in their standard states. This value is crucial for thermodynamic calculations and helps predict the energy changes associated with chemical reactions. It is typically found in tables and is used to calculate the overall enthalpy change for reactions involving the formation of compounds.
Recommended video:
Guided course

Enthalpy of Formation
Related Practice
Textbook Question
Textbook Question
What is the value of the standard enthalpy of formation of an element in its most stable form?
Textbook Question
For each of the following compounds, write a balanced thermochemical equation depicting the formation of one mole of the compound from its elements in their standard states and then look up H °f for each substance in Appendix C. (b) FeCl3(s)
Textbook Question
Many portable gas heaters and grills use propane, C3H8(g), as a fuel. Using standard enthalpies of formation, calculate the quantity of heat produced when 10.0 g of propane is completely combusted in air under standard conditions.
Textbook Question
Using values from Appendix C, calculate the value of H for each of the following reactions: (a) NiO(s) + 2 HCl(g) → NiCl2(s) + H2O(g)
