What is the value of the standard enthalpy of formation of an element in its most stable form?

The following is known as the thermite reaction: 2 Al(s) + Fe2O3(s) → Al2O3(s) + 2 Fe(s). This highly exothermic reaction is used for welding massive units, such as propellers for large ships. Using standard enthalpies of formation in Appendix C, calculate _x001F_H ° for this reaction.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Thermodynamics and Enthalpy

Standard Enthalpy of Formation

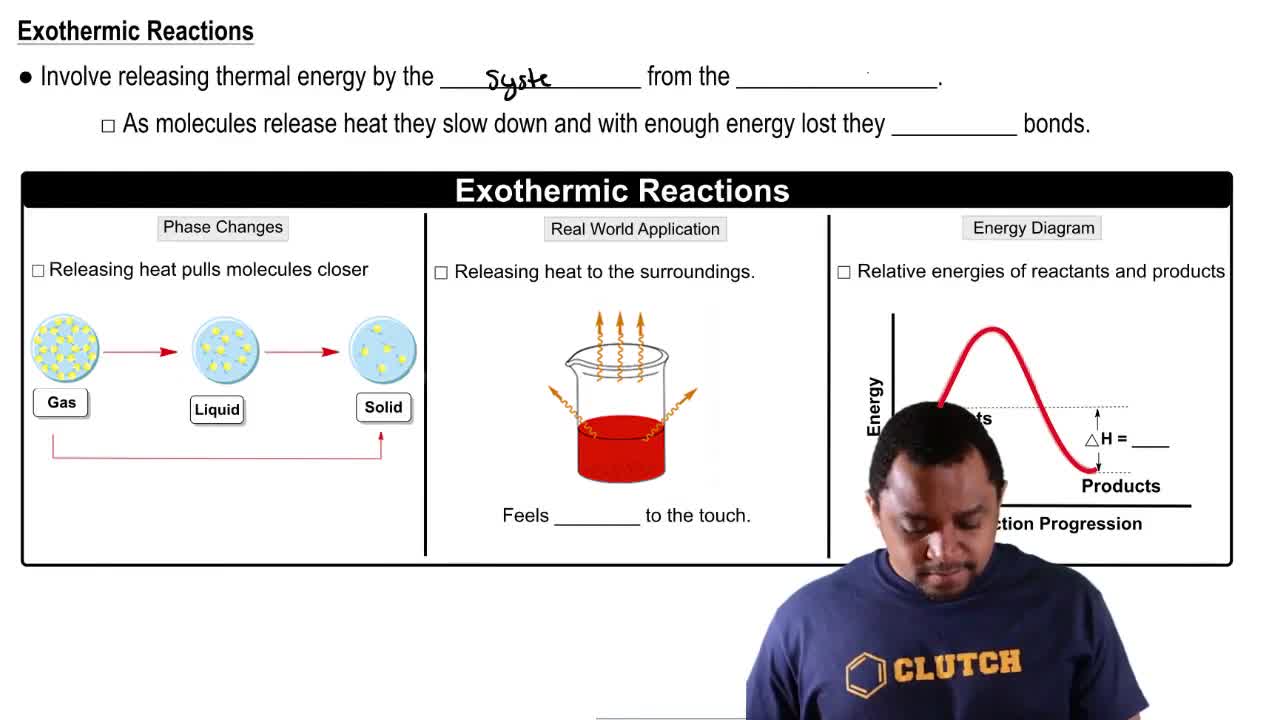
Exothermic Reactions
For each of the following compounds, write a balanced thermochemical equation depicting the formation of one mole of the compound from its elements in their standard states and then look up H °f for each substance in Appendix C. (b) FeCl3(s)
Write balanced equations that describe the formation of the following compounds from elements in their standard states, and then look up the standard enthalpy of formation for each substance in Appendix C: (a) NH4NO3(s)
Many portable gas heaters and grills use propane, C3H8(g), as a fuel. Using standard enthalpies of formation, calculate the quantity of heat produced when 10.0 g of propane is completely combusted in air under standard conditions.
Using values from Appendix C, calculate the value of H for each of the following reactions: (a) NiO(s) + 2 HCl(g) → NiCl2(s) + H2O(g)
Complete combustion of 1 mol of acetone (C3H6O) liberates 1790 kJ: C3H6O(l) + 4 O2(g) → 3 CO2(g) + 3 H2O(l) ΔH° = -1790 kJ Using this information together with the standard enthalpies of formation of O2(g), CO2(g), and H2O(l) from Appendix C, calculate the standard enthalpy of formation of acetone.
