
(c) It turns out that the difference in energies between the valence atomic orbitals of H and F are sufficiently different that we can neglect the interaction of the 1s orbital of hydrogen with the 2s orbital of fluorine.
The 1s orbital of hydrogen will mix only with one 2p orbital of fluorine. Draw pictures showing the proper orientation of all three 2p orbitals on F interacting with a 1s orbital on H. Which of the 2p orbitals can actually make a bond with a 1s orbital, assuming that the atoms lie on the z-axis?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Atomic Orbitals
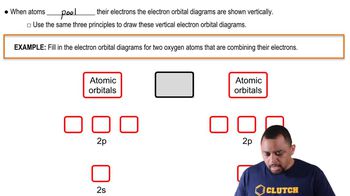
Orbital Hybridization

Molecular Orbital Theory

Azo dyes are organic dyes that are used for many applications, such as the coloring of fabrics. Many azo dyes are derivatives of the organic substance azobenzene, C12H10N2. A closely related substance is hydrazobenzene, C12H12N2. The Lewis structures of these two substances are
(Recall the shorthand notation used for benzene.) (c) Predict the N¬N¬C angles in each of the substances.
(b) How many of the MOs from part (a) would be occupied by electrons?
Carbon monoxide, CO, is isoelectronic to N2. (d) Would you expect the p2p MOs of CO to have equal atomic orbital contributions from the C and O atoms? If not, which atom would have the greater contribution?
The energy-level diagram in Figure 9.36 shows that the sideways overlap of a pair of p orbitals produces two molecular orbitals, one bonding and one antibonding. In ethylene there is a pair of electrons in the bonding π orbital between the two carbons. Absorption of a photon of the appropriate wavelength can result in promotion of one of the bonding electrons from the p2p to the p*2p molecular orbital. (a) Assuming this electronic transition corresponds to the HOMO-LUMO transition, what is the HOMO in ethylene?
The energy-level diagram in Figure 9.36 shows that the sideways overlap of a pair of p orbitals produces two molecular orbitals, one bonding and one antibonding. In ethylene there is a pair of electrons in the bonding π orbital between the two carbons. Absorption of a photon of the appropriate wavelength can result in promotion of one of the bonding electrons from the p2p to the p*2p molecular orbital. (b) Assuming this electronic transition corresponds to the HOMO-LUMO transition, what is the LUMO in ethylene?
