
Azo dyes are organic dyes that are used for many applications, such as the coloring of fabrics. Many azo dyes are derivatives of the organic substance azobenzene, C12H10N2. A closely related substance is hydrazobenzene, C12H12N2. The Lewis structures of these two substances are

(Recall the shorthand notation used for benzene.) (c) Predict the N¬N¬C angles in each of the substances.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
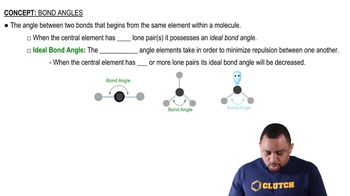
Key Concepts
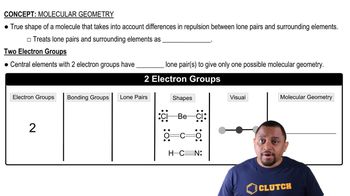
Lewis Structures

Molecular Geometry
Bond Angles
Place the following molecules and ions in order from smallest to largest bond order: N22+, He2+, Cl2 H2-, O22-.
Azo dyes are organic dyes that are used for many applications, such as the coloring of fabrics. Many azo dyes are derivatives of the organic substance azobenzene, C12H10N2. A closely related substance is hydrazobenzene, C12H12N2. The Lewis structures of these two substances are
(Recall the shorthand notation used for benzene.) (b) How many unhybridized atomic orbitals are there on the N and the C atoms in each of the substances? How many unhybridized atomic orbitals are there on the N and the C atoms in hydrazobenzene?
(b) How many of the MOs from part (a) would be occupied by electrons?
(c) It turns out that the difference in energies between the valence atomic orbitals of H and F are sufficiently different that we can neglect the interaction of the 1s orbital of hydrogen with the 2s orbital of fluorine.
The 1s orbital of hydrogen will mix only with one 2p orbital of fluorine. Draw pictures showing the proper orientation of all three 2p orbitals on F interacting with a 1s orbital on H. Which of the 2p orbitals can actually make a bond with a 1s orbital, assuming that the atoms lie on the z-axis?
