Textbook Question
Draw the structure of all isomers of the octahedral complex [NbX2Cl4]- (X- = NCS-), and identify those that are linkage isomers.

 Verified step by step guidance
Verified step by step guidance
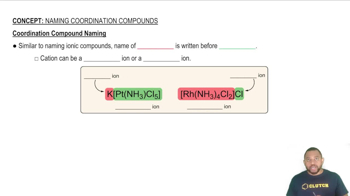
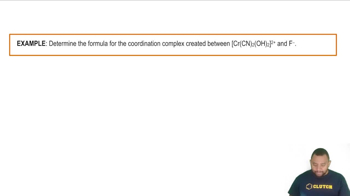

Draw the structure of all isomers of the octahedral complex [NbX2Cl4]- (X- = NCS-), and identify those that are linkage isomers.
Classify the following ligands as monodentate, bidentate, tri-dentate, or tetradentate. Which can form chelate rings?
(a)
(b)
(c)
(d)
What is a racemic mixture? Does it affect plane-polarized light? Explain.
Which of the following complexes can exist as enantiomers? Draw their structures.
(a) [Cr(en)3]3+
(b) cis-[Co(NH3)Cl]2+
(c) trans-[Co(en)2(NH3)Cl]2+
(d) [Pt(NH3)3Cl3]+
Predict the number of unpaired electrons for each of the following.
(a) Sc3+
(b) Co2+
What is the highest oxidation state for each of the elements from Sc to Zn?