Ch.12 - Solids and Modern Materials

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 12, Problem 81
(a) What is a monomer? (b) Which of these molecules can be used as a monomer: ethanol, ethene (also called ethylene), methane?
 Verified step by step guidance
Verified step by step guidance1
Understand the definition of a monomer: A monomer is a molecule that can undergo polymerization, binding chemically to other molecules to form a polymer.
Identify the functional groups or characteristics that allow a molecule to act as a monomer. Typically, monomers have double bonds or reactive groups that can form bonds with other monomers.
Analyze each molecule: Ethanol (C_2H_5OH) is an alcohol with a hydroxyl group, which is not typically reactive enough to form polymers through addition polymerization.
Consider ethene (C_2H_4), which has a carbon-carbon double bond. This double bond can open up and link with other ethene molecules, making it a suitable monomer for addition polymerization.
Evaluate methane (CH_4), which is a saturated hydrocarbon with single bonds only, making it generally unreactive for polymerization.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Monomers and Polymers
Monomers are small, simple molecules that can chemically bond to form larger structures known as polymers. In polymer chemistry, monomers serve as the building blocks for creating various materials, such as plastics and fibers. Understanding the distinction between monomers and polymers is essential for identifying which substances can participate in polymerization reactions.
Recommended video:
Guided course
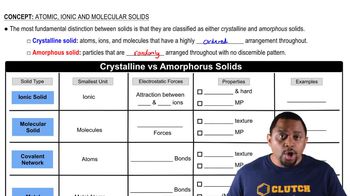
Crystalline vs Amorphous Solids
Types of Hydrocarbons
Hydrocarbons are organic compounds consisting entirely of hydrogen and carbon. They can be classified into alkanes, alkenes, and alkynes based on the types of bonds between carbon atoms. Ethene (ethylene) is an alkene with a double bond, making it a suitable candidate for polymerization, while ethanol and methane do not have the same reactivity for forming long-chain polymers.
Recommended video:
Guided course
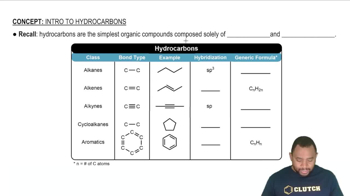
Intro To Hydrocarbons
Polymerization Process
Polymerization is the chemical process through which monomers link together to form polymers. There are two main types: addition polymerization, where unsaturated monomers like alkenes react to form a polymer, and condensation polymerization, which involves the elimination of small molecules like water. Recognizing the conditions and types of monomers that can undergo polymerization is crucial for determining their suitability as monomers.
Recommended video:
Guided course

Spontaneity of Processes
Related Practice
