Textbook Question
Name each carboxylic acid or ester.

 Verified step by step guidance
Verified step by step guidance
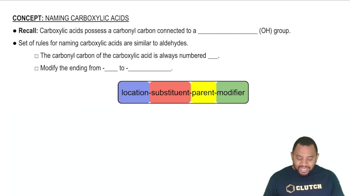
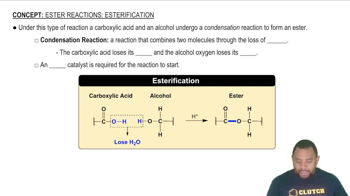

Name each carboxylic acid or ester.
Name each carboxylic acid or ester.
Name each carboxylic acid or ester.
Draw the structure of each carboxylic acid or ester. b. methyl hexanoate
Draw the structure of each carboxylic acid or ester. c. 3-ethylheptanoic acid
Draw the structure of each carboxylic acid or ester.
d. butyl ethanoate