Textbook Question
The accompanying diagram shows how ΔH (red line) and TΔS (blue line) change with temperature for a hypothetical reaction.
(b) In what temperature range is this reaction spontaneous?

 Verified step by step guidance
Verified step by step guidance
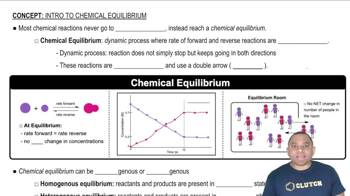


The accompanying diagram shows how ΔH (red line) and TΔS (blue line) change with temperature for a hypothetical reaction.
(b) In what temperature range is this reaction spontaneous?
Consider a reaction A2(𝑔) + B2(𝑔) ⇌ 2 AB(𝑔), atoms of A shown in red in the diagram and atoms of B shown in blue. (b) If 𝐾𝑐 = 1, which box represents the system at 𝑄 < 𝐾𝑐?
Which of the following processes are spontaneous and which are nonspontaneous: (a) the ripening of a banana