Isomers are molecules that have the same chemical formula but different arrangements of atoms, as shown here for two isomers of pentane, C5H12.
(a) Do you expect a significant difference in the enthalpy of combustion of the two isomers? Explain.

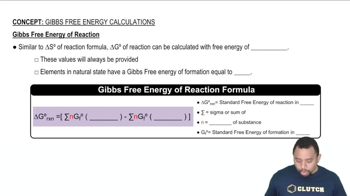
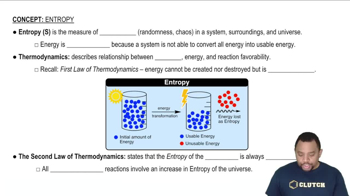
 Verified step by step guidance
Verified step by step guidance

Isomers are molecules that have the same chemical formula but different arrangements of atoms, as shown here for two isomers of pentane, C5H12.
(a) Do you expect a significant difference in the enthalpy of combustion of the two isomers? Explain.
Isomers are molecules that have the same chemical formula but different arrangements of atoms, as shown here for two isomers of pentane, C5H12.
(b) Which isomer do you expect to have the higher standard molar entropy? Explain.
Consider a reaction A2(𝑔) + B2(𝑔) ⇌ 2 AB(𝑔), atoms of A shown in red in the diagram and atoms of B shown in blue. (a) If 𝐾𝑐 = 1, which box represents the system at equilibrium?
Consider a reaction A2(𝑔) + B2(𝑔) ⇌ 2 AB(𝑔), atoms of A shown in red in the diagram and atoms of B shown in blue. (b) If 𝐾𝑐 = 1, which box represents the system at 𝑄 < 𝐾𝑐?