At ordinary body temperature (37 °C), the solubility of N2 in water at ordinary atmospheric pressure (1.0 atm) is 0.015 g/L. Air is approximately 78 mol % N2. (b) At a depth of 100 ft in water, the external pressure is 4.0 atm. What is the solubility of N2 from air in blood at this pressure?
Ch.13 - Properties of Solutions

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 13, Problem 109b
A series of anions is shown below:

The anion on the far right is called 'BARF' by chemists, as its common abbreviation sounds similar to this word. (b) What is the electron-domain geometry around the central B in BARF?
 Verified step by step guidance
Verified step by step guidance1
Identify the central atom in the BARF anion, which is Boron (B).
Determine the number of electron domains around the central Boron atom. In this case, Boron is bonded to four fluorine atoms.
Recall that electron-domain geometry considers both bonding pairs and lone pairs of electrons around the central atom.
Since Boron has four bonding pairs and no lone pairs, the electron-domain geometry is based on four electron domains.
The electron-domain geometry for four electron domains is tetrahedral.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electron-Domain Geometry
Electron-domain geometry refers to the spatial arrangement of electron groups around a central atom in a molecule. These groups can include bonding pairs of electrons, lone pairs, and single electrons. The geometry is determined by the number of these electron groups, which influences the molecular shape and angles between bonds.
Recommended video:
Guided course
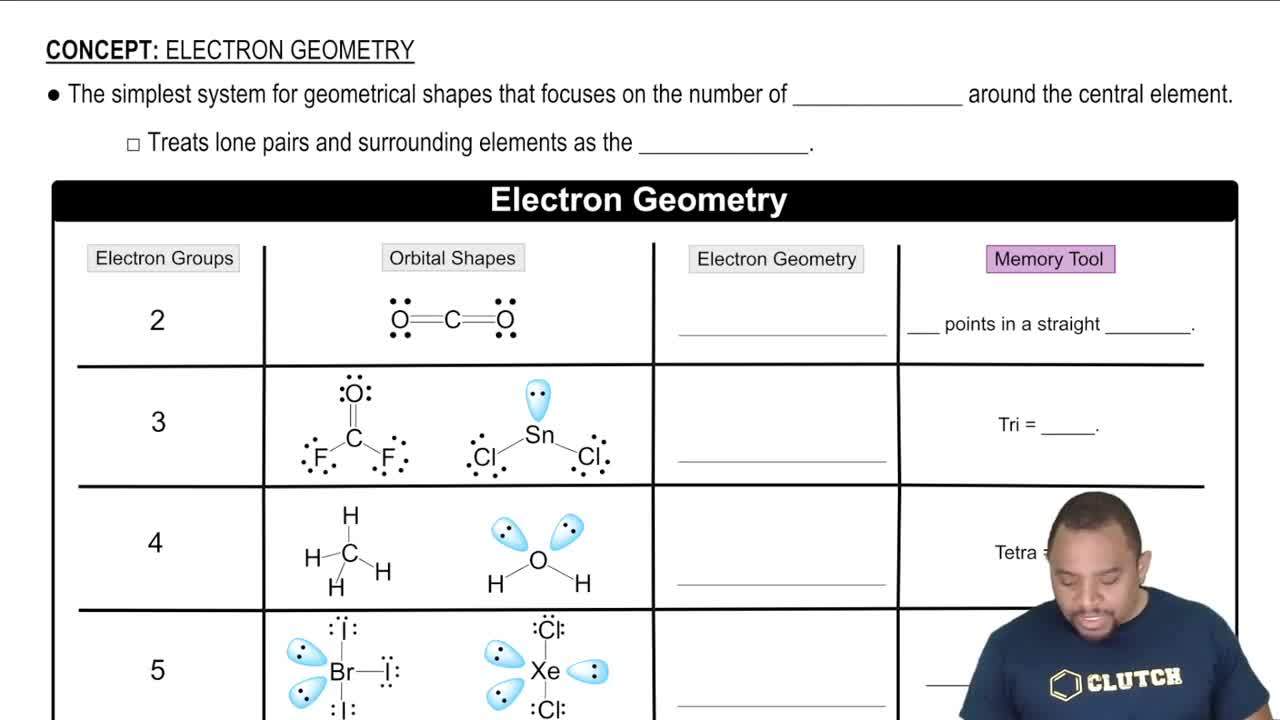
Electron Geometry
VSEPR Theory
Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the geometry of individual molecules based on the repulsion between electron pairs in the valence shell of the central atom. According to VSEPR, electron pairs will arrange themselves as far apart as possible to minimize repulsion, leading to specific geometrical shapes such as linear, trigonal planar, or tetrahedral.
Recommended video:
Guided course
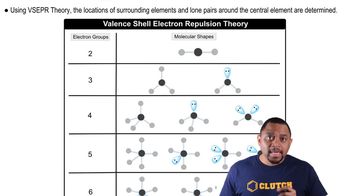
Molecular Shapes and VSEPR
Central Atom Hybridization
Hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals that can accommodate bonding. The type of hybridization (e.g., sp, sp², sp³) of the central atom affects the electron-domain geometry. For example, sp³ hybridization corresponds to a tetrahedral geometry, while sp² leads to trigonal planar geometry, which is crucial for determining the shape of the BARF anion.
Recommended video:
Guided course
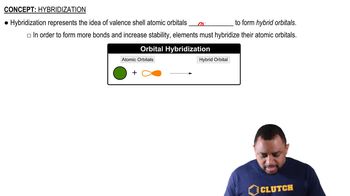
Hybridization
Related Practice
Textbook Question
Textbook Question
A series of anions is shown below:The anion on the far right is called 'BARF' by chemists, asits common abbreviation sounds similar to this word.(d) Tetrabutylammonium, (CH3CH2CH2CH2)4N + is a bulky cation. Which anion, when paired with the tetrabutylammonium cation, would lead to a salt that will be most soluble in nonpolar solvents?
Textbook Question
A series of anions is shown below:
The anion on the far right is called 'BARF' by chemists, as its common abbreviation sounds similar to this word. (a) What is the central atom and the number of electronpair domains around the central atom in each of these anions?
2
views
Textbook Question
A series of anions is shown below: The anion on the far right is called 'BARF' by chemists, as its common abbreviation sounds similar to this word. (c) Which, if any, of these anions has an expanded octet around its central atom?
Textbook Question
A small cube of lithium 1density = 0.535 g/cm32 measuring 1.0 mm on each edge is added to 0.500 L of water. The following reaction occurs: 2 Li1s2 + 2 H2O1l2 ¡ 2 LiOH1aq2 + H21g2 What is the freezing point of the resulting solution, assuming that the reaction goes to completion?
