Hydrogen sulfide is an impurity in natural gas that must be removed. One common removal method is called the Claus process, which relies on the reaction: 8 H2S1g2 + 4 O21g2¡S81l2 + 8 H2O1g2 Under optimal conditions the Claus process gives 98% yield of S8 from H2S. If you started with 30.0 g of H2S and 50.0 g of O2, how many grams of S8 would be produced, assuming 98% yield?

Write the balanced chemical equations for c. the combination reaction between nickel metal and chlorine gas.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Combination Reaction
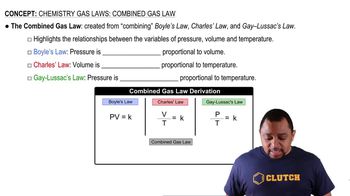
Balancing Chemical Equations

Chemical Symbols and Formulas
When hydrogen sulfide gas is bubbled into a solution of sodium hydroxide, the reaction forms sodium sulfide and water. How many grams of sodium sulfide are formed if 1.25 g of hydrogen sulfide is bubbled into a solution containing 2.00 g of sodium hydroxide, assuming that the sodium sulfide is made in 92.0% yield?
Write the balanced chemical equations for a. the complete combustion of acetic acid (CH3COOH), the main ingredient in vinegar;
The effectiveness of nitrogen fertilizers depends on both their ability to deliver nitrogen to plants and the amount of nitrogen they can deliver. Four common nitrogen-containing fertilizers are ammonia, ammonium nitrate, ammonium sulfate, and urea [(NH2)2CO] Rank these fertilizers in terms of the mass percentage nitrogen they contain.
b. How many molecules of C9H8O4 are in this tablet?
(b) Hemoglobin, the oxygen-carrying protein in red blood cells, has four iron atoms per molecule and contains 0.340% iron by mass. Calculate the molar mass of hemoglobin.
